Recurrent scrotal edema in liver cirrhosis
Liver cirrhosis is a potentially lethal disease, which can be complicated by liver failure, hepatic encephalopathy, splenomegaly, variceal bleeding, ascites, and hydrothorax (1,2). Herein, we reported a rare case with liver cirrhosis presenting with recurrent scrotal edema probably due to an extremely albumin level.
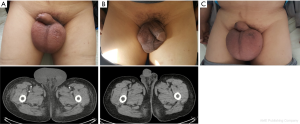
On March 18, 2017, a 62-year-old male with a previous history of hepatitis B virus related liver cirrhosis was complaint of progressive edema of scrotum at our department. He denied any history of cardiac, renal, or thromboembolic diseases. On physical examinations, he had moderate jaundice, an enlarged scrotum of 10 cm × 8 cm (Panel A), negative shifting dullness, mildly enlarged spleen, and moderate edema of both lower limbs. Contrast-enhanced computed tomography scans demonstrated mild pleural effusion, shrunken and distorted liver, mild ascites, and splenomegaly. No thromboembolic diseases were found. Hepatitis B virus surface antigen and e antibody IgG were positive. Hepatitis B virus DNA viral load was 3.6×106 IU/mL (reference range: <1.0×103 IU/mL). On laboratory tests, total bilirubin was 107.9 μmol/L (reference range: 5.1–22.2 μmol/L), albumin was 15.8 g/L (reference range: 40–55 g/L), prothrombin time was 30.8 seconds (reference range: 11.5–14.5 seconds), international normalized ratio was 2.96, NT-proBNP was 268.4 pg/mL (reference range: 0–125 pg/mL), and serum creatine was 77 μmol/L (reference range: 44–133 μmol/L). Child-Pugh score was 12. Model for end-stage liver disease (MELD) score was 26. Intravenous infusion of albumin with oral diuretic and antiviral drugs were given. Nine days later, scrotal edema remarkably disappeared (Panel B). On March 27, 2017, laboratory tests demonstrated that total bilirubin was 130.1 μmol/L, albumin was 31.1 g/L, prothrombin time was 32.5 seconds, international normalized ratio was 3.17, NT-proBNP was 598.4 pg/mL, and serum creatine was 92.75 μmol/L. Child-Pugh score was 11. MELD score was 27. And then he refused hospitalization and liver transplantation due to poor economic status.
On June 9, 2017, he was re-admitted to our department due to recurrent edema of scrotum. On physical examinations, the shifting dullness was negative, and the volume of scrotum became larger (Panel C). Hepatitis B virus surface antigen and e antibody IgG remained positive. Hepatitis B virus DNA viral load was 2.3×104 IU/mL. Laboratory tests demonstrated that total bilirubin was 168.7 μmol/L, albumin was 18.5 g/L, prothrombin time was 28.4 seconds, international normalized ratio was 2.58, NT-proBNP was 712.1 pg/mL, and serum creatine was 118.0 μmol/L. MELD score was 30. He developed the disturbance of consciousness after his hospitalization. On June 9, 2017, his relatives refused hospitalization. On June 26, 2017, he died at home.
Scrotal edema, a rare sign, has been incidentally reported in patients with inferior vena cava thrombus (3), dilated cardiomyopathy (4), end-stage heart failure (5), juvenile dermatomyositis (6), Henoch-Schönlein purpura (7), acute pancreatitis (8), cancer of penis (9), and radiation enteritis (10), etc. In children, scrotal edema is often idiopathic and self-limiting with a low rate of recurrence (11). To our knowledge, few studies reported scrotal edema as a complication of liver cirrhosis (12-17). A scrotal edema might be produced on the basis of the communications between the peritoneal and subcutaneous spaces. As previously reported, scrotal edema might be iatrogenic in most of cirrhotic cases. Some cases developed after abdominal paracentesis (14,15), and others after portacaval anastomosis (16) or laparoscopic cholecystectomy (17). By comparison, the present case might be attributed to an extremely low albumin level. After albumin supplementation and diuretics, scrotal edema disappeared.
In conclusion, we reported a rare case with liver cirrhosis presenting with scrotal edema secondary to hypoalbuminemia. Additionally, recurrent scrotal edema might be an ominous sign for cirrhotic patients’ outcomes.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.08.22). Xingshun Qi serves as an Editor-in-Chief of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Djiambou-Nganjeu H. Hepatic Encephalopathy in Liver Cirrhosis. J Transl Int Med 2017;5:64-7. [Crossref] [PubMed]
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749-61. [Crossref] [PubMed]
- Ucer O, Nese N, Muezzinoglu T. Pure Yolk sac presenting with inferior vena cava thrombus extending from bilateral external iliac veins to hepatic vein. Int Braz J Urol 2016;42:1244-7. [Crossref] [PubMed]
- Fabregat-Andrés O, Coret-Moya M, Ridocci-Soriano F. Scrotal edema in dilated cardiomyopathy. Acta Med Port 2014;27:146. [Crossref] [PubMed]
- Sabar R, Safadi W. Relieving the burden: palliative centesis of an oedematous scrotal wall due to anasarca in end-stage heart failure. BMJ Case Rep 2013;2013.
- Sallum AM, Silva MF, Michelin CM, et al. Penile and scrotum swelling in juvenile dermatomyositis. Acta Reumatol Port 2011;36:176-9. [PubMed]
- Croche Santander B, Campos E, Sanchez A, et al. Arch Argent Pediatr 2016;114:e249-51. [Henoch-Schonlein purpura involving the penis: a case report]. [PubMed]
- Moens L, Yengue Yengue P, Assenmacher C. Intrascrotal Collection in an Acute Pancreatitis: A Case Report and Review of the Literature. Case Rep Urol 2016;2016:7534781.
- Chen HK, Sinagra D, Vivian J. Scrotal oedema in a man who had cystoprostatectomy and ileal diversion 9 years ago. BMJ Case Rep 2014;2014.
- Fan S, Chen Y, Wang J, et al. Recurrent scrotal edema in a patient with radiation enteritis: A case report. Mol Clin Oncol 2016;5:79-82. [Crossref] [PubMed]
- Santi M, Lava SAG, Simonetti GD, et al. Acute Idiopathic Scrotal Edema: Systematic Literature Review. Eur J Pediatr Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Schnarkowski P, Burkard R, Wolff M. Scrotal swelling in a young male with chronic alcoholism. Invest Radiol 1993;28:770-3. [Crossref] [PubMed]
- Hou F, Qi X, Li H, et al. Scrotal Edema in a Patient with Liver Cirrhosis. Austin J Gastroenterol 2016;3:1066.
- Conn HO. Sudden scrotal edema in cirrhosis: a postparacentesis syndrome. Ann Intern Med 1971;74:943-5. [Crossref] [PubMed]
- Pereira W, Seeff LB. Sudden scrotal edema:old pearls? Ann Intern Med 1971;75:647. [Crossref] [PubMed]
- Conn HO. Unilateral edema and jaundice after portacaval anastomosis. Ann Intern Med 1972;76:459-61. [Crossref] [PubMed]
- Geffre M, Maki C, Maier S. Acute Scrotal Edema in Cirrhotic after Laparoscopic Cholecystectomy. Am Surg 2017;83:93-5. [PubMed]
Cite this article as: Qi X, An S, Li H, Guo X. Recurrent scrotal edema in liver cirrhosis. AME Med J 2017;2:124.