Ruijin robotic thoracic surgery: robot-assisted Ivor Lewis esophagectomy
Clinical data
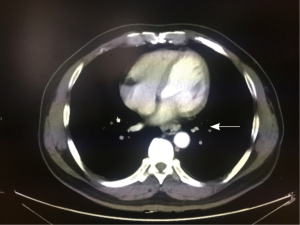
A 51-year-old man was admitted to our hospital with a 1-week history of progressive dysphagia with solid food. He did not complain of retrosternal pain, gastroesophageal reflux or, weight loss. Esophagogastroscopy identified a 3-cm mass in the esophageal lumen approximately 35 cm from the incisors, which was diagnosed as squamous cell carcinoma by endoscopic biopsy. Computed tomography (CT) of the chest and the abdomen revealed a thick wall around the distal thoracic esophagus with no metastases in the liver or lung, and the lymph nodes were negative (Figure 1). Barium swallow demonstrated a filling defect in the lumen of the distal third of the esophagus. Physical examination revealed no abnormalities. His cardiopulmonary function and laboratory tests were normal. He had no medical history.
Operation steps
Anesthesia and body position
Abdominal phase
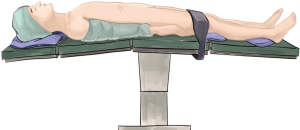
After the general anesthesia and double-lumen endotracheal intubation, the patient was placed in a supine position (Figure 2).
Thoracic phase
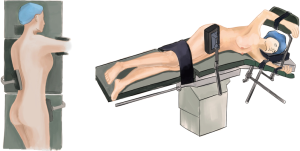
Once the abdominal phase was completed, the patient was positioned in the left lateral decubitus position, and tilted 45° towards the prone position under double-lumen endotracheal intubation (Figure 3).
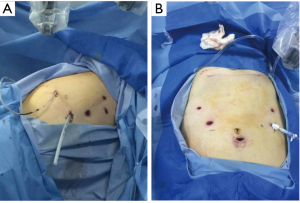
Ports
Abdominal phase
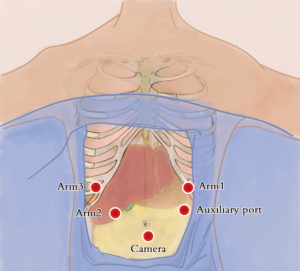
Abdominal ports: the five-port method was used. The subumbilical port was used for observation (12-mm trocar), the #1 robotic arm was placed on the left anterior axillary line under the costal arch (8-mm trocar), the #2 robotic arm was placed on the right anterior axillary line at the umbilical level (8-mm trocar), and the manual operative port was placed on the right mid clavicular line at 3 cm under the costal arch (12-mm trocar). An auxiliary port was placed on the left anterior axillary line at the umbilical level (8-mm trocar) (Figure 4).
Thoracic phase
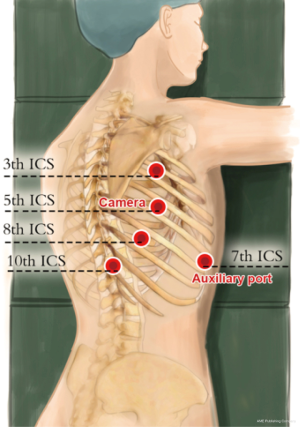
Thoracic ports: the five-port method was used. The observation port was placed on the right anterior axillary line at the 5th intercostal space (12-mm trocar), the #1 robotic arm was placed on right posterior axillary line at the 3th intercostal level (8-mm trocar), the #2 robotic arm was placed on the right posterior axillary line at 8th intercostal space (8-mm trocar), and the manual operative ports were placed on the right posterior axillary line at the 10th (5-mm trocar), and an auxiliary port were placed on the right anterior axillary line at 7th intercostal spaces (12-mm trocar) (Figure 5).
Installation of the surgical arms
Abdominal phase
The #2 arm was connected to a bipolar cautery forceps, and the #1 arm was connected to an ultrasound knife.
Thoracic phase
The robot was positioned on the dorsal cranial side, with two assistants on the anterior side. The #2 arm was connected to a bipolar cautery forceps, and the #1 arm was connected to a unipolar cautery hook.
Surgical procedure
Abdominal phase
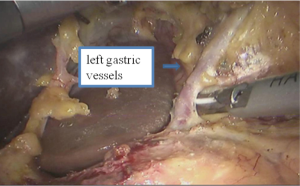
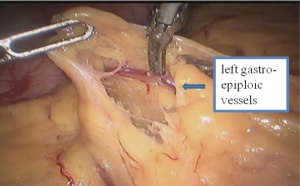
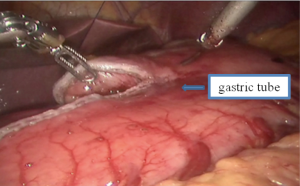
Thoracic phase
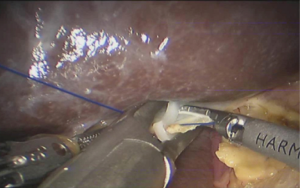
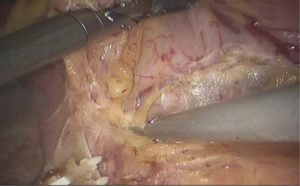
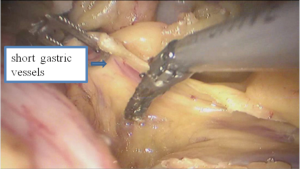
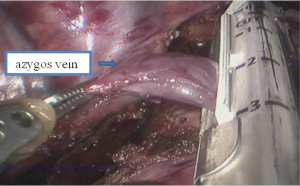
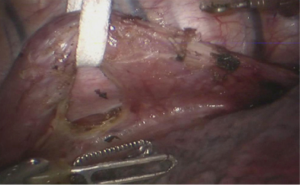
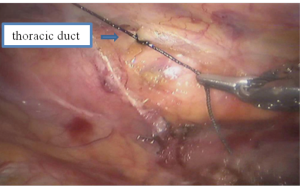
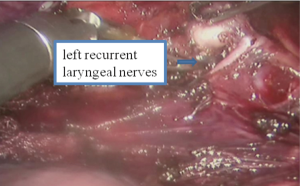
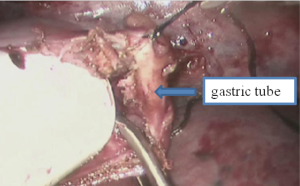
See Figures 13,14,15,16,17,18,19,20,21,22.
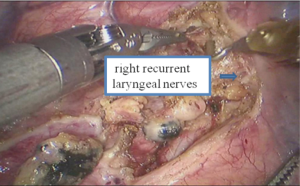
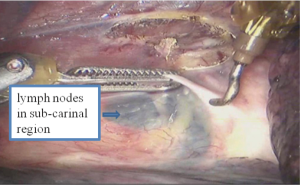
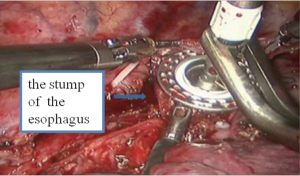
Postoperative condition
Postoperative treatments included anti-inflammatory medication, enteral nutrition and phlegm-resolving treatment. The chest cavity drainage tube was withdrawn after 2 days and the liquid diet was started on postoperative day 6. The patient was discharged on postoperative day 8 and tolerated a semi-liquid diet. No complications were observed during hospitalization. Pathologic diagnosis was squamous cell carcinoma infiltrating into the submucosa of the esophagus. All lymph nodes were negative. Postoperative pathologic stage was pT1N0M0 (IA squamous cell carcinoma).
Discussion
Surgery is currently the main treatment for esophageal cancer (1). Esophagectomy is technically challenging and is associated with high morbidity and mortality rates. Efforts to reduce these rates have spurred the adoption of minimally invasive techniques (2). But the conventional video-assisted surgery has some limitations such as the two-dimensional view or movement restrictions which could make a complex procedure such as esophagectomy difficult. Robotic systems have been designed to overcome some of these disadvantages which could provide an amplified three-dimensional view and a greater freedom of movement (3). Most of the published reports on robotic esophagectomy describe two types of anastomosis including cervical or intrathoracic anastomosis that are created by using the suturing technique (4,5). Here we report the robot-assisted Ivor Lewis esophagectomy with intrathoracic stapled anastomosis. Our initial results suggest that the robotic-assisted surgical technique is safe and satisfies the oncological principles. However, the potential of the da Vinci system remains to be proven in future clinical trials.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.01.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dimick JB, Goodney PP, Orringer MB, et al. Specialty training and mortality after esophageal cancer resection. Ann Thorac Surg 2005;80:282-6. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Jeffrey Yang CF, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- Park SY, Kim DJ, Yu WS, et al. Robot-assisted thoracoscopic esophagectomy with extensive mediastinal lymphadenectomy: experience with 114 consecutive patients with intrathoracic esophageal cancer. Dis Esophagus 2016;29:326-32. [Crossref] [PubMed]
- Hodari A, Park KU, Lace B, et al. Robot-Assisted Minimally Invasive Ivor Lewis Esophagectomy With Real-Time Perfusion Assessment. Ann Thorac Surg 2015;100:947-52. [Crossref] [PubMed]
Cite this article as: Zhang Y, Yang S, Guo W, Jin R, Chen X, Wu H, Du H, Han D, Chen K, Xiang J, Li H. Ruijin robotic thoracic surgery: robot-assisted Ivor Lewis esophagectomy. AME Med J 2017;2:3.