Postoperative management of robotic-assisted thoracic surgery
Introduction
With the widely application of robot-assisted thoracic surgery using the da Vinci Surgical System, the delivery of specialized nursing care has come to the forefront. Robot-assisted thoracic surgery with less trauma, less bleeding, and quick recovery has reduced the difficulty of clinical nursing care and increase the expectation of an enhanced recovery after surgery.
Mental care
Patient unfamiliar with the da Vinci Surgical System may have unreasonable expectations or a skeptical attitude. Short-term discomfort after surgery may prevent patients for seeing the advantages of da Vinci surgery. In addition, patients and their families may be disappointed in the seemingly minor benefits over conventional surgery, particularly as this procedure is more expensive. In addition to preoperative health education, nurses should offer professional postoperative health education, emphasizing the features and benefit of da Vinci surgery versus conventional surgery, to boost patient confidence. Nurses also set up a group for patients undergoing da Vinci surgery to share their feelings, which will ultimately improve patient outcomes.
Diet is mainly divided into two categories according to type of disease and surgery
- For non-gastrointestinal surgeries (e.g., pulmonary disease, mediastinal disease), the diet should be bland and consist of easily digested semi-liquids (e.g., porridge, noodles, ravioli), gradually increasing the intake of protein, calories, and vitamins. Provide a high-roughage diet for regular defecation.
- For gastrointestinal surgeries (e.g., esophageal disease, cardiac disease), patients should receive enteral nutrition the first day after surgery. The nutrient solutions should be given at the appropriate temperature (37–42 °C) in the appropriate amount (gradually increasing from 500 to 1,500–2,000 mL/d), and at an appropriate rate (gradually increasing from 50 to 120 mL/h). Adjust fluid speed and amount of nutrient solution according to the chief complaints of the patients. Typically, the postoperative diet starts 24 hours after stopping gastrointestinal decompression in the absence of dyspnea, chest pain, ipsilateral diminished breath sounds, fever, or other symptoms of anastomotic fistula. Patients usually start with a little water before advancing to a liquid diet and then a semiliquid diet. About 1 month after the surgery, patients are able to advance to a soft diet. Patients should eat smaller more frequent meals, chew foods thoroughly, eat and drink slowly, and avoid raw or cold foods to prevent anastomotic fistula. Sitting in a reclining position for 2 hours after meals and using high pillows while sleeping can prevent reflux.
Position
After surgery the patient should take a semi-reclining position (the head of the bed should be elevated 30°) to ease breathing and drainage and minimize coughing, sputum, and pain. After 6 hours, patients can move into other positions with the assistance of nurses after eating or fluid infusion.
Pain care
- Keep the ward quiet. Ensure the patients get sufficient rest. Help the patient find a comfortable position.
- Teach the patients how to move in bed. Fix the thoracic drainage tube while the patient is changing position or coughing to avoid irritating the pleura and consequent chest pain.
- Instruct patients to relax by using abdominal deep breaths or soothing music.
- Give analgesics according to the doctors’ instruction and watch for signs of respiratory depression.
- Encourage early removal of the thoracic drainage tube to avoid the pain caused by factors such as the traction of the duct due to the lower amount of effusion of da Vinci surgery.
Airway management
- Closely observe respiratory patterns (frequency and rhythm) and perform pulmonary auscultation to see if the breath sounds are normal and whether there are signs of hypoxia.
- Encourage the patients to take deep breaths or use a respiration training device to promote lung expansion. Help the patients expectorate. Pat them on the back (on both sides of the spinal column, from basis pulmonis to apex pulmonis, from bottom to top, from outside to inside, about 2–3 times per second) or use auxiliary instruments. Compress the trachea or take thyrocricocentesis to inject saline, if needed, to induce cough.
- Use a nebulizer to dilute the sputum and make it easier to cough.
- Use a fiberoptic bronchoscope to suction sputum for patients who are too weak to cough.
Tubes care
- Thoracic drainage tube (1)
- To ensure effective drainage, observe whether the pipe column fluctuates with respiration. Squeeze the tube to prevent obstruction of the drainage tube if there are blood clots or floccules in it.
- Place the drainage bottle lower than the incision.
- Properly place and fix the drainage bottle to prevent tilting and determine the appropriate length of the drainage tube to facilitate the patients’ activities and prevent distortion.
- Observe and record the color, quality, and quantity of the fluids and gas discharge in the drainage tube. Note that bleeding >200 mL/h for 3 hours indicates active hemorrhage.
- Use strict aseptic technique.
- Maintain the tightness of the drainage tube. Use two clamps to seal the duct while moving the patients or changing the drainage bottle to prevent air from entering. Avoid pneumothorax or atelectasis caused by clamping the duct when there is air leakage. Ask the patients to exhale and use Vaseline, gauze, and tape to protect the wound and inform the doctor if the thoracic drainage tube slips.
- Indications of extubation include 24–72 hours’ drainage without gas discharge or with dwindling clear fluids, drainage fluids <100 mL/d, pus <10 mL/d, chest X-ray with good lung expansion, and no signs of dyspnea.
- Jejunal fistula and nasogastric feeding tube
- The tube must be tied or taped securely, and the patients should take a semi-reclining position to prevent reflux and aspiration. Extra caution is required when the patients has a choking cough because of the possibility of reflux or aspiration. Patients should be encouraged to cough to discharge inhaled fluid. Use a fiberoptic if needed.
- The skin around and under the ties or tube should be frequently assessed for ulcerations. Lubricate the nasal mucosa with paraffin oil daily.
- The tube needs periodical flushing to remain unobstructed.
- Pause injection of the jejunal nutrient solution and inform the doctor if there is exudation or slipping.
- Gastric tube
- The tube must be tied properly to prevent slipping.
- Monitor the quality and quantity of drainage fluids. A small amount of bloody or coffee-like fluid can be aspirated from the gastric tube 6–12 hours after the surgery, and then the drainage fluids should become clearer. A large amount of bloody fluid, irritability, decreased blood pressure, increased pulse rate, and decreased urine volume suggest anastomotic bleeding that needs emergency treatment.
- Squeeze the tube to keep it unobstructed and use saline if needed.
- If slipping occurred, do not reinsert the gastric tube to prevent piercing the anastomosis and cause anastomotic leakage.
Complications
The da Vinci surgical system has been proven to be an effective procedure with enhanced visualization, better dexterity, and fewer complications. In addition to common thoracic surgery complications, the following complications may occur due to the characteristics of da Vinci robotic system.
- Postoperative hemorrhage (2-5): the da Vinci robotic system lacks tactile feedback, which may cause undetectable intraoperative hemorrhage due to excessive traction of tissues and blood vessels. Members of the healthcare team must pay attention to the patients’ vital signs, chief complaints, and drainage fluids.
- Hypercapnia: CO2 pneumoperitoneum during surgery may cause acidosis, subcutaneous emphysema, and other complications. Nurses should carefully observe the patients’ state of consciousness and respiratory rate and enhance airway management to promote CO2 removal.
Exercise and rehabilitation
Injuries to blood vessels, muscles, and nerves adjacent to the surgical field are inevitable. Patients may experience shoulder stiffness, muscle atrophy, and upper extremity dysfunction due to postoperative pain. Early ambulation can prevent complications such as shoulder joint ankylosis, disuse atrophy, atelectasis, pressure ulcers, constipation, and deep venous thrombosis (6).
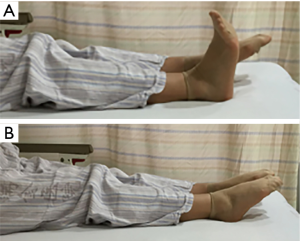
- Early in-bed mobilization: patients can start to clench their fists or do ankle pump exercises (Figure 1) (holding for 5–10 seconds and then relaxing 3 times a day) once they awake from anesthesia. These exercises may promote blood circulation to improve limb numbness and stimulate lower extremity venous reflux to prevent deep venous thrombosis.
- Shoulder exercises (Figure 2):
- Patients can start finger flexion and extension or ankle pump exercises (3–5 minutes, 3 times a day) once they awaken from anesthesia.
- Patients can start elbow flexion and extension movement the first day after surgery. They may use their ipsilateral hand to bush teeth, wash face, and eat. To comb the hair, the patient should maintain the neck in a neutral position and raise the elbow. Raise the hand above the head and use the contralateral hand to drag the ipsilateral elbow. Put the ipsilateral hand on the shoulder, try to touch the contralateral ear, and gradually put the ipsilateral hand over the head. Do each exercise described above for 3–5 minutes, 3 times a day.
- Patients can start comprehensive exercise including arm movement the second day after surgery. The ipsilateral and contralateral sides should work together. Lift arms together. Lift arms alternately. Flap arms. Cross fingers behind the head. Open and close elbows in front of the chest at the same height and then open backwards. Do each exercise described above for 3–5 minutes, 3 times a day.
- Out-of-bed activities: nurses should assist the patients with steady vital signs in out-of-bed activities the first day after surgery.
- Preparation: fix the drainage tubes and take the drainage bottle. Keep drainage tubes lower than the incision to prevent reflux.
- For the patients who are in a supine or Fowler’s position: support the body with the upper limbs against the bed to move to the side of the bed. For patients with good pain tolerance, lie on the side with help, and sit up at the bedside with the support of a single arm. For patients with poor pain tolerance, sit up with the help of an assistant lifting the neck. Ask the patients if there is dizziness or discomfort. Sit up 30 seconds after waking up, stand up 30 seconds after sitting up, and start walking 30 seconds after standing up. Patients may have cough because of the position change. Pause walking until they expel the sputum with the help of healthcare providers.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.01.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Luo Y, Han T. Postoperative nursing of mediastinal tumors resection patients assisted by Da Vinci-S surgery. Journal of Clinical Medicine in Practice 2015;19:47-9.
- Kong P, Xu P, et al. Postoperative nursing of robot-assisted thymectomy in 36 patients. Journal of Nursing (China) 2013;20:29-31.
- Jing R, Zhang H, Zhao Y, et al. 161 cases nursing experience of preoperative period about robotic assisted radical hysterectomy. Practical Journal of Clinical Medicine 2015;12:12930.
- Tong X, Xu S, Wang S, et al. Clinical experience of the treatment of solitary pulmonary nodules with Da Vinci surgical system. Zhongguo Fei Ai Za Zhi 2014;17:541-4. [PubMed]
- Herron DM, Marohn MSAGES-MIRA Robotic Surgery Consensus Group. A consensus document on robotic surgery. Surg Endosc 2008;22:313-25; discussion 311-2. [Crossref] [PubMed]
- Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001;322:473-6. [Crossref] [PubMed]
Cite this article as: Hu Y, Pu J, Wu B, Chen X, Li H. Postoperative management of robotic-assisted thoracic surgery. AME Med J 2017;2:11.