Budd Chiari syndrome presenting with hepatic transient mosaic enhancement pattern and treated with early transjugular intrahepatic portosystemic shunt
Key points
- Budd Chiari syndrome (BCS) can present with ascites and transient hepatic mosaic enhancement pattern on CT imaging.
- TIPS is an established interventional treatment of BCS.
- Timing of TIPS for BCS is a debated issue.
- Early interventional treatment for BCS could challenge the Step-Wise management.
Case presentation
Ascites can be the main sign at presentation in many different diseases and aetiology is sometimes not easy to recognize. Hepatic sinusoidal dilatation, due to impaired hepatic outflow or to both inflammatory or infective abdominal diseases, on CT and MR imaging typically appears with the so-called mosaic enhancement pattern (MEP), defined as reticulated enhancement on contrast-enhanced acquisitions during the arterial-dominant and/or the portal venous phase that disappears during the delayed phase (1). Recently, diverse acute extrahepatic, both abdominal and not, infectious and inflammatory diseases have been reported to be cause of transient MEP (2). We report a case of BCS presenting with ascites and transient MEP and treated with early transjugular intrahepatic portosystemic shunt (TIPS). This paper does not rely on an experimental but on an already established and effective treatment. Consequently, we obtained the informed consent of the patient but we did not require any ethics committee approval.
A 73-year-old man presented at a community hospital because of persistent ascites since 6 months before. Comorbidities were mild chronic renal failure (mean creatinine 1.3 mg/dL), arterial hypertension and previous occasional finding of multiple mediastinal lymph nodes (max 2 cm), unchanged for 6 months on CT imaging. There was not history of alcohol intake either evidence of HCV nor HBV infection. Moreover, autoantibodies were negative. Abdominal US was normal apart from ascites. Peritoneal fluid was a transudate and negative for both neoplastic cells and TBC. There were not clinical nor echocardiographic signs of congestive heart failure nor constrictive pericarditis.
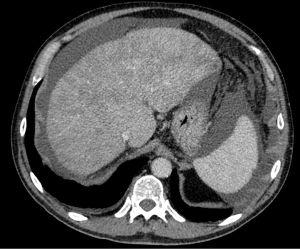
On CT imaging there was moderate ascites, diffuse hepatic MEP during portal phase, no evidence of hepatic nor peritoneal cancer and apparently normal flows in hepatic veins (HV), inferior vena cava (IVC) and portal vein (PV) (Figure 1).
Due to a hypothesis of BCS a complete work-up was performed without evidence of any pro-thrombotic disorder. Then, after a 2 L paracentesis, treatment with coumadin and furosemide (40 mg day) was started.
Two months later, because of ascites persistence, he was admitted at our department. Physical examination showed moderate ascites and abdominal subcutaneous portosystemic shunts. Biochemistry was the following: WBC 6,900/mmc, Hb 13 g/dL, PLT 170,000/mmc, glucose 111 mg/dL, urea 28 mg/dL, creatinine 1.2 mg/dL, Na 138 mEq/L, K 4.1 mEq/L, AST 31 U/L, ALT 35 U/L, GGT 57 U/L, ALP 111 U/L, bilirubin 1 mg/dL, INR 2, protein 6.9 g/L, albumin 4.2 g/L, gamma globulin g/L 1.1, urinary Na 60 mEq/L. Endoscopy showed F1 esophageal varices. Doppler US reported ascites and apparently normal flows in HV, IVC and PV but tortuous course of middle and right HV, suggesting intrahepatic collateral circulation.
CT, performed two months after the former, confirmed absence of hepatic nor peritoneal cancer, moderate ascites, normal flows in HV, IVC and PV, but disappearance of hepatic MEP in portal phase.
Consequently, transjugular liver biopsy was performed and histology confirmed a picture of BCS.
Few days later, after a 4 L paracentesis, TIPS was performed with PTFE-covered stent between right HV and PV right branch. There were not complications after TIPS procedure.
Thirty months after TIPS, clinical conditions were excellent. Biochemistry was the following: WBC 6,900 mmc, Hb 13 g/dL, PLT 170,000 mmc, glucose 111 mg/dL, urea 60 mg/dL, creatinine 1.8 mg/dL, Na 138 mEq/L, K4.1 mEq/L, AST 31 U/L, ALT 35 U/L, GGT 57 U/L, ALP 111 U/L, bilirubin 1 mg/dL, INR 2, protein 6.9 g/L, albumin 4.2 g/L, gamma globulin 1.1 g/L. There was no ascites nor signs of TIPS dysfunction at Doppler US, nor episodes of hepatic encephalopathy had been reported. Mediastinal lymph nodes were still unchanged at CT. Total body PET did not show evidence of neoplasia.
Discussion
Hepatic MEP is expression of sinusoidal dilatation, described in BCS, other vascular disorders (PV thrombosis, congenital absence of the PV, obliterative portal venopathy), cardiac/pericardial diseases and diverse chronic inflammatory disorders (e.g., Castleman’s disease, Crohn’s disease, sarcoidosis, rheumatoid arthritis, HIV). Furthermore, different acute extrahepatic infectious and inflammatory diseases both abdominal and extra-abdominal, like pyelonephritis, pancreatitis, pneumonia, septicaemia, Crohn’s disease, infectious colitis, have be reported to be cause of transient MEP. In such cases, MEP was often during both arterial and portal phase, always diffuse but more conspicuous in subcapsular areas, and in all resolved after resolution of the acute disease. In fact, no liver biopsy was indicated (2).
We report a case of transient MEP due to BCS in which, despite the resolution of MEP on CT imaging, ascites persisted. The case underlines both the diagnostic challenge of BCS and the realistic underestimation of HV thrombosis in BCS on imaging, an important issue not only for diagnostic work-up, but also for the choice of treatment (3). In fact, in our case, disappearance of MEP during oral anticoagulant therapy despite persistence of clinical ascites suggests that HV thrombosis extension does not necessarily correlate with BCS severity and, as recent data show, that medical therapy alone is generally not sufficiently effective as treatment of BCS (3-13).
BCS is a rare but severe thrombotic disease affecting HV and/or IVC and impairing hepatic outflow. BCS is generally due to prothrombotic disorders (14,15). However, the fact that in our case no prothrombotic disorder is coherent with recent data confirming that an amount of BCS lacks a triggering disease (16).
Regarding management of BCS, both AASLD and, with irrelevant differences, EASL guidelines suggest a step-wise strategy with medical therapy the first-line treatment, angioplasty/stenting as second-line, TIPS the next step and liver transplant (LT) the last one (14,15); and the indication of moving forward when no response to therapy appears. However, definition for response to therapy was taken from that used in a study on only 51 patients, relies on arbitrary clinical and biochemical data, was not stated and needs validation (17). The proposed criteria are the following: clinical failure to therapy (treatment failure) was considered when criteria for complete or ongoing response were lacking. Complete response was considered when all of the following six criteria were met and stable: (I) absence of clinically detectable ascites, with normal serum sodium and creatinine levels, in the absence of diuretic therapy, or on low dose diuretics (spironolactone 75 mg/d or furosemide 40 mg/d) and moderate NaCl intake; (II) increase in coagulation factor V to a level above 40% of normal value; (III) decrease in conjugated serum bilirubin to a level below 15 mmol/L; (IV) absence of first or recurrent portal hypertension related bleeding while on primary or secondary prophylaxis with non-selective beta blockers or with endoscopic therapy; (V) no occurrence of spontaneous bacterial infection; and (VI) BMI >20 kg/m2 after substraction of ascites and edema. Ongoing response was considered when all of the following three criteria were met: (I) in the presence of ascites, a negative sodium and water balance was achieved using low dose diuretics and moderate sodium intake, together with normal serum sodium and creatinine levels, or with increasing serum sodium if initially low and decreasing serum creatinine levels if initially high; (II) factor V level was increasing if initially low; and (III) serum conjugated bilirubin level was decreasing if initially high (18).
Interestingly, if the management of our case had followed these criteria, our patient would have been treated with only medical therapy (instead of TIPS, as he was).
Furthermore, the outcome of patients with BCS treated with the step-wise strategy depends on such criteria (14,15).
In fact, the widest long-term multicenter experience on BCS reported that while the overall long-term survival of patients treated using the step-wise strategy was good, almost 30% of patients who received only medical therapy died, a high rate considering the availability of effective invasive treatments (14). Moreover, a recent systematic review confirmed unacceptable increased mortality in BCS on sole medical therapy (10).
Furthermore, studies both from West and China recently reported further data in favour or early TIPS for BCS (11,12).
The biggest criticism of both the AASLD and EASL guidelines seems the assumption that BCS should receive further treatment only when hemodynamic consequences of portal hypertension become clinically evident, such giving scarse consideration to the chronic ischemic liver damage effects on hepatic function and to the possibility of preventing liver failure by relieving impaired HV outflow (15,16).
Fibrogenesis has a central role on BCS physiopathology and a substantial rate of patients has histological cirrhosis. Moreover, recent data have shown that chronic hepatic congestion leads to sinusoidal thrombosis and strain, which promote hepatic fibrosis (19).
Furthermore, clinical complications of BCS likely become evident after months or years HV thromboses have instituted in most of the cases, as either abdominal venous and/or subcutaneous collaterals are often evident at diagnosis (3). Progression from a phase to another is probably related to both temporally subsequent thrombotic events and to hepatic functional reserve (3). Consequently, a reasoning model of BCS physiopathology is that not only impaired hepatic vein outflow has hemodynamic consequences on portal hypertension development, but also causes hepatic fibrosis and liver failure through chronic ischemic liver damage (3).
Recently, with the aim of preventing hepatic fibrosis development, disease progression, and finally improving outcome, I reported a proposal of the management of BCS, in which medical therapy is suggested only for patients without any sign of portal hypertension, whether early interventional treatment when signs of portal hypertension appear (3).
The choice of interventional treatment between angioplasty/stent, TIPS or surgery, in patients considered unsuitable for only medical therapy, generally addressed due to local skillness, is another central issue. TIPS should probably be the first choice, being the mostly used and a standardized and homogeneous technique. Moreover, TIPS can possibly be effective in case of thrombosis extension to the portal tree. Furthermore, it gives assurance of an adequate outflow and hepatic congestion relieve regardless of the length of the stenoses (3). The second choice should be angioplasty/stent or surgery. The former should be preferred in case of short-length stenoses, the latter is particularly useful in cases of high IVC thrombosis unsuitable for TIPS with the benefit of avoiding LT (3). Finally, LT has to be taken in consideration only as rescue treatment (3,15,16).
In conclusion, BCS is a difficult diagnosis. Moreover, transient hepatic MP may be indicative of BCS. Finally, Early Interventional Treatment for BCS could challenge Step-Wise Management.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.05.12). Andrea Mancuso serves as an unpaid editorial board member of AME Medical Journal from Mar 2017 to Mar 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang DM, Jung DH, Park CH, et al. Imaging findings of hepatic sinusoidal dilatation. AJR Am J Roentgenol 2004;183:1075-7. [Crossref] [PubMed]
- Ronot M, Kerbaol A, Rautou PE, et al. Acute extrahepatic infectious or inflammatory diseases are a cause of transient mosaic pattern on CT and MR imaging related to sinusoidal dilatation of the liver. Eur Radiol 2016;26:3094-101. [Crossref] [PubMed]
- Mancuso A. An Update on Management of Budd-Chiari Syndrome: the Issues of Timing and Choice of Treatment. Eur J Gastroenterol Hepatol 2015;27:200-3. [Crossref] [PubMed]
- Mancuso A. An Update on Management of Budd-Chiari syndrome. Ann Hepatol 2014;13:323-6. [PubMed]
- Mancuso A. TIPS for Budd-Chiari syndrome: Time to anticipate treatment. Liver International 2014;34:e325. [Crossref] [PubMed]
- Mancuso A. Budd-Chiari Syndrome management: Timing of treatment is an open issue. Hepatology 2014;59:1213. [Crossref] [PubMed]
- Mancuso A, Fung K, Mela M, et al. TIPS for acute and chronic Budd-Chiari syndrome: a single-centre experience. J Hepatol 2003;38:751-4. [Crossref] [PubMed]
- Mancuso A, Watkinson A, Tibballs J, et al. Budd-Chiari syndrome with portal, splenic, and superior mesenteric vein thrombosis treated with TIPS: who dares wins. Gut 2003;52:438. [Crossref] [PubMed]
- Mancuso A. Time to resize the role of liver transplant for Budd-Chiari syndrome. Liver Int 2015;35:2339. [Crossref] [PubMed]
- Qi X, Ren W, Wang Y, et al. Survival and prognostic indicators of Budd-Chiari sybdrome: a systematic review of 79 studies. Expert Rev Gastroenterol Hepatol 2015;9:865-75. [PubMed]
- Rosenqvist K, Sheikhi R, Eriksson LG, et al. Endovascular treatment of symptomatic Budd-Chiari syndrome – in favour of early transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol 2016;28:656-60. [Crossref] [PubMed]
- He F, Zhao H, Dai S, et al. Transjugular intrahepatic portosystemic shunt for Budd-Chiari syndrome with diffuseocclusion of hepatic veins. Sci Rep 2016;6:36380. [Crossref] [PubMed]
- Mancuso A. Limits of Evidence Based Medicine for rare diseases: The case of Budd-Chiari syndrome. Dig Liver Dis 2016;48:689. [Crossref] [PubMed]
- Seijo S, Plessier A, Hoekstra J, et al. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology 2013;57:1962-8. [Crossref] [PubMed]
- DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology 2009;49:1729-64. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol 2016;64:179-202. [Crossref] [PubMed]
- Qi X, Han G, Guo X, et al. Review article: the aetiology of primary Budd-Chiari syndrome - differences between the West and China. Aliment Pharmacol Ther 2016;44:1152-67. [Crossref] [PubMed]
- Plessier A, Sibert A, Consigny Y, et al. Aiming at minimal invasiveness as a therapeutic strategy for Budd-Chiari syndrome. Hepatology 2006;44:1308-16. [Crossref] [PubMed]
- Simonetto DA, Yang HY, Yin M, et al. Chronic passive venous congestion drives hepatic fibrogenesis via sinusoidal thrombosis and mechanical forces. Hepatology 2015;61:648-59. [Crossref] [PubMed]
Cite this article as: Mancuso A, Butera G, Politi F, Maringhini A. Budd Chiari syndrome presenting with hepatic transient mosaic enhancement pattern and treated with early transjugular intrahepatic portosystemic shunt. AME Med J 2017;2:60.