Lumbar spinal stenosis: an update on the epidemiology, diagnosis and treatment
IntroductionOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
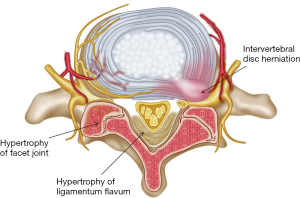
Lumbar spinal stenosis (LSS) is a common spinal disorder in the older population, and a clinical syndrome consisting of pain in the buttock or lower extremity, with or without low back pain and corresponding imaging findings of narrowing of spaces around neural and vascular elements in the lumbar spine (1-3). The narrowing factors could be the intervertebral disc herniation, hypertrophy of ligamentum flavum, hypertrophy of facet joint, spondylolisthesis, osteophyte and ectopic fat tissue (Figures 1,2).
EpidemiologyOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
The exact prevalence of LSS is still unknown. It is estimated that more than 200,000 adults are affected by LSS in the United States (2), and will rise to 64 million elderly adults by the year 2025 (4). The Framingham Study (5) found that congenital relative LSS was 4.7% and absolute LSS was 2.6%, acquired relative and absolute LSS was 22.5% and 7.3%, respectively, for 60–69 years old population, the relative and absolute LSS was 47.2% and 19.4%, respectively. A population-based study in Japan (6) found that the LSS incidence was increased by age, about 1.7–2.2% in 40–49 years old population, and 10.3%–11.2% in 70–79 years old population. Another study reported the incidence of symptomatic LSS is about 10% (7). The LSS is the most common reason for >65 years old patients to undergo the spinal surgery (8). During 2002 to 2007, the rate of lumbar stenosis surgery per 100,000 Medicare beneficiaries is about 135.5–137.5 persons, the mean hospital charges for decompression alone is about $23,724 and combined with fusion is $80,888, and in 2009, the hospital bill for LSS for Medicare beneficiaries was $1.65 billion (9), which is a significant socioeconomic burden.
History and symptomsOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
Neurogenic claudication is the most common symptom for LSS patients. The patients complain of pain or discomfort that radiates to the buttock, thigh and lower leg after walking for a certain distance (10,11), therefore leading to functional disability and decreased walking capacity (12,13). For some dynamic components in LSS patients, the pain symptom often is relieved at the position of sitting down or lumbar flexion (using a shopping cart or bicycle), and exacerbated at the position of lumbar extension, which will reduce the area of lumbar spinal canal (14-17). Patients with lumbar stenosis with spondylolisthesis often have low back pain (18,19), and other symptoms include leg numbness, imbalance and lower extremity weakness (12,20).
Physical examinationOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
Physical examination includes the assessment of gait (normal or wide-based gait), modified Romberg maneuver (the patients’ feet are kept together and eyes closed for about 10 seconds and observed for imbalance), no pain with flexion, strength of knee flexors and extensors, ankle dorsiflexors and plantar flexors, pinprick sensation and achilles reflex. The specificity of wide-based gait, abnormal Romberg result is more than 90%, but the sensitivity is less than 50%, the sign of “No pain with flexion” has a sensitivity of 79%, only 44% for specificity (16). None of the above physical examination has higher percentage in both sensitivity and specificity.
Radiographic imagesOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
Plain radiography
Spondylolisthesis can be observed from plain radiographic images, but not all spondylolisthesis will lead to LSS. Some other signs of LSS include narrowing height of intervertebral foramina and space of intervertebral disc, small interlaminar window, hypertrophy of facet joint, short pedicles, thick lamina, and deep posterior concavity of vertebral bodies (21). The plain radiographic image is only helpful for some obvious stenosis or spondylolisthesis.
CT and MRI
MRI is commonly used to confirm the LSS. The MRI, which has excellence in observation for soft tissue, is recommended to diagnose LSS by many authors (22-24). CT will be used for some patients with suspected ossification, or if MRI is contraindicated, or unavailable.
Although there is no gold standard quantitative criteria in MRI to diagnose the LSS, currently, several parameters are used clinically, the most common ones for central LSS are anteroposterior canal diameter, cross sectional area; for lateral LSS, the most common ones are the height and depth of the lateral recess and the lateral recess angle; for foraminal stenosis, they are foraminal diameter and height, hypertrophic facet joint degeneration and foraminal nerve root impingement (25,26). It is reported that anteroposterior canal diameter <10–15 mm, the cross sectional area <75–145 mm2 as the cut-off values to definition of central stenosis (27-37) (Figure 3), the details of them are shown in Table 1. It is reported that the height/depth of lateral recess ≤2–5 mm, and angle of lateral recess <30° to define the lateral stenosis (38-40) (Figure 4), the details of them are showed on Table 2.
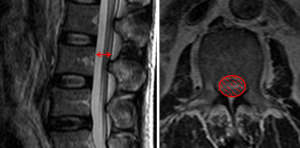
Table 1
| Literatures | Anteroposterior canal diameter (mm) | Cross sectional area (mm2) |
|---|---|---|
| CT | ||
| Ullrich (32) | <11.5 | <145 |
| Haig (27) | ≤11.95 | – |
| Bolender (33) | <13 | 100–130 (early stenosis); <100 (present stenosis) |
| Lee (34) | <15 (suggesting narrowing); <10 (usually diagnostic) | – |
| Verbiest (35) | <12 (relative); <10 (absolute) | – |
| Schönström (36) | – | <100 |
| Schönström (37) | – | 75–100 (moderate); <75 (severe) |
| MRI | ||
| Fukusaki (28) | <15 | – |
| Koc (29) | <12 | – |
| Mariconda (31) | – | <130 |
| Hamanishi (30) | – | <100 |
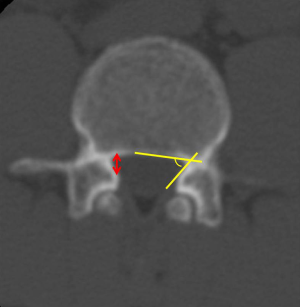
Table 2
In a large-scale MRI study (41) with standardized measurements to determine the clinical MRI criteria for developmental LSS, the results suggest the developmental LSS can be defined if the anteroposterior canal diameter was at L1 <20 mm, L2 <19 mm, L3 <19 mm, L4 <17 mm, L5 <16 mm, and at S1 <16 mm.
Another radiographic sign—“sedimentation sign”—the lack of sedimentation of the nerve roots to the dorsal part of the dural sac (positive sedimentation sign) in MRI is recognized as a reliable sign to diagnose LSS (42-45). A recent meta-analysis (46) including seven studies found that the sensitivity of sedimentation sign is 0.80 (95% CI: 0.77–0.83) and the specificity is 0.96 (95% CI: 0.94–0.98). In patients with severe morphological LSS, the sedimentation sign had even higher sensitivity of 0.899 (95% CI: 0.87–0.92) and specificity of 0.99 (95% CI: 0.98–1.00). It was also reported that patient with positive sedimentation sign may had greater surgical treatment effect and as an informed treatment choice regarding surgery for LSS (47,48), the reversibility of a pre-operative positive sedimentation sign after surgery was associated with an improved clinical outcome, however, the persisting post-operative positive sedimentation sign could be the result of incomplete decompression or surgical complications (49).
Myelography
It has been found that myelography had a slightly higher accuracy in diagnosing LSS than CT (33), Bell et al. reported that the accuracy of myelography was 93% vs. 89% by CT (50). MRI and myelography may have similar accuracy in diagnosing lumbar canal stenosis (51), in study of Bischoff et al. (52), they found that myelography was the most specific diagnostic method (with specific 88.9%) when compared to the myelo-CT and MRI in diagnosis of lumbar canal stenosis. However, because of the drawbacks of invasiveness and relative side effects of myelography, it is not commonly used in clinical practice.
Additional investigations
The electromyography is not routinely used in clinic, however, for some special clinical conditions where the radiographic images cannot explain the symptoms of patients, or unilateral symptoms with bilateral pathology (53), or if there is a need to differentiate between LSS and peripheral neuropathy (54), electromyography may be helpful. Caution needs to be taken if there is co-existing LSS and peripheral neuropathy. A quantitative electromyographic technique—paraspinal mapping—may be useful in diagnosis of LSS, and reflects physiology of nerve roots better than the limb electromyography (55). Selective lumbar nerve root block can be used to identify the responsible level for some patients with multiple anatomic LSS, and may reduce the levels of operation (56,57).
Some other techniques of magnetic stimulation caudal motor conduction time (58), dermatomal somatosensory-evoked potentials (59,60) are rarely used, and their accuracy remains uncertain.
Diagnosis and differential diagnosisOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
No gold standard diagnostic criterion is widely accepted among physicians (22,61). Therefore, to diagnose LSS, we need comprehensive consideration of the history, physical examination, and radiographic images. Most often information including age, neurogenic claudication, the radiating buttock or leg pain which is exacerbated when lumbar extension and relieved at seat or lumbar flexion, wide based gait, and the anatomic narrow observed at radiographic images, sometimes even the results of electromyography and nerve root block is used.
Common conditions that need to be included in the differential diagnoses include vascular claudication, peripheral neuropathy, hip osteoarthritis and trochanteric bursitis. The symptomatic presentation of a shopping cart sign, symptoms located above the knees, triggered with standing alone and relieved with sitting had a strong correlation with neurogenic claudication, while symptoms in calf and relieved with standing alone is related to vascular claudication (62), and peripheral neuropathy patients may have history of diabetes mellitus (63). Electromyography may help the differential diagnosis (64,65). Hip osteoarthritis and trochanteric bursitis are also common in elderly patients, selective anesthetic and corticosteroid injection at hip joint or trochanteric bursa may help the differential diagnosis. Nonetheless, the above diseases may co-exist with LSS, making it complicated to differentiate occasionally.
ClassificationOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
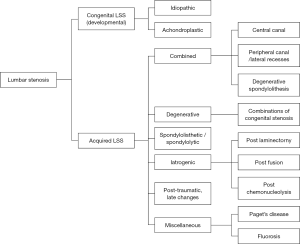
Many different classifications have been reported, but there is a lack of an accepted classification system. One of the most common classification systems was described by Arnoldi et al. (66), which categorized the LSS into congenital LSS and acquired LSS. Congenital LSS includes idiopathic and achondroplastic LSS, and acquired LSS including degenerative, combined, spondylolisthesis, iatrogenic, post-traumatic and miscellaneous. The details of the Arnoldi classification are summarized in Figure 5.
TreatmentOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
Non-operative
Many non-operative options can be selected for LSS patients, including lifestyle modifications, drugs, physiotherapy, multidisciplinary rehabilitation, epidural injections and some complementary medicine. There is still a lack of high quality randomized controlled trials to prove the efficacy of non-operative methods (67,68). Lifestyle changes such as weight loss, quit smoking may decrease the incidence of low back pain (69), improve walking capacity and quality of life (70). The prescriptions of over-the-counter (OTC) drugs include gabapentin, vitamin B1 and prostaglandins, non-steroidal anti-inflammatory drugs (NSAIDs) may provide some symptomatic relief. Recent systematic reviews found (71,72) that oral NSAIDs are more effective than placebo and acetaminophen for persistent low back pain, intramuscular NSAIDs have similar outcomes as combined manipulation and soft tissue therapy. No evidence suggests that calcitonin administration has benefit in pain relief or walking distance improvement for patients with LSS (73,74). Opioid may be prescribed for some severe pain patients, however, long-term opioid use had higher risk, operative option may better for them (75).
Physiotherapy including many different kinds of treatments, such as massage or manipulation, exercises (strengthening exercises and flexibility exercises), balance training, wear braces or corset), pain management by heat, ice, electrical stimulation, some lifestyle modification and complementary medicine (acupuncture) also could be included in physiotherapy category (76). The exercises may have short-term benefit for leg pain and function compared with no treatment, but the quality of evidence is low (68). Currently, there is no evidence to show that one kind of treatment is superior to the others (77).
Epidural injections may include local anesthetic injection with or without steroids. The epidural injections may result in some improvement in radicular pain in short term (78-80), improvement in pain and functional parameters seen similar between epidural injections and physical therapy (29). Researchers also found that epidural injection of combinations of anesthetic and steroids has no beneficial effect compared with epidural anesthetic alone injection (28,81). Moreover, epidural injection does not have any impact on average impairment of function, risk of need for surgery, or provide long-term pain relief (78-80).
Operative
Surgical intervention is recommended if the symptoms are persistent. There are several kinds of operative techniques, including decompression alone, interspinous spacers, and spinal arthrodesis.
Decompression
The aim of the decompression is to decompress the spinal canal and foramina, remove the pressure factors and release the nerve roots. The decompression approaches include conventional laminectomy, bilateral laminotomy, unilateral laminotomy with contralateral recess decompressed by transmedia way (Figure 6), partial facetectomy and split-spinous process laminotomy/laminoplasty (82-86). Decompression can significantly relieve the symptoms of claudication and radicular leg pain, improve the physical function for LSS patients (87,88). A prospective 10-year study comparing surgical decompression with conservative treatment found excellent or fair results in half of conservative patients and in four-fifths of surgical decompression patients after a period of 4 years, and the treatment result for the patients randomized for surgical treatment was considerably better than for the patients randomized for conservative treatment (89).
One randomized controlled study (RCT) (90) included a total of 94 patients with 50 operative and 44 nonoperative patients found both operative and nonoperative managements showed improvement of pain and Oswestry Disability Index (ODI), but the mean difference in favor of operation was 11.3 in disability (95% CI: 4.3–18.4), 1.7 in leg pain (95% CI: 0.4–3.0), and 2.3 (95% CI: 1.1–3.6) in back pain at one year follow up and 7.8 in disability (95% CI: 0.8–14.9) 1.5 in leg pain (95% CI: 0.3–2.8), and 2.1 in back pain (95% CI: 1.0–3.3) at two year follow up. Walking ability, either reported or measured, did not differ between the two different treatments. Another clinical trial included 289 randomized assigned patients and 365 non-randomized assigned patients showed a significant treatment effect favoring surgery on the SF-36 scale for bodily pain and no significant difference in ODI in randomized assigned patients. When combining the randomized and non-randomized assigned patients, the ones who underwent surgery showed more significantly improvement in pain scores and ODI than non-operative ones (87).
A Cochrane systematic review found that: compared to the conventional laminectomy, the bilateral laminotomy may be better in perceived recovery, the unilateral laminotomy for bilateral decompression and bilateral laminotomy may have lower incidence of iatrogenic instability, and the bilateral laminotomy and split-spinous process laminotomy may have less severity of postoperative low back pain (84).
Interspinous spacer
For the theory of dynamic component in LSS patients, and the pain relieved at the position of sitting down or lumbar flexion, exacerbated at lumbar extension (14-16), many interspinous spacers (such as X-stop, coflex, DIAM, and Aperius devices) were designed and used in clinic (91-93). Biomechanical study (94) showed the interspinous spacer significantly increased the canal area by 18%, subarticular diameter by 50%, canal diameter by 10%, the foraminal area by 25%, and the foraminal width by 41% in extension. Therefore, interspinous spacer is an alternative choice for LSS nowadays (95), it can be inserted percutaneously alone without decompression or combined with open or microsurgical decompression. The percutaneous stand-alone spacer implantation has advantages of being minimally invasive (96,97), however, may have high risk of un-satisfactory back pain, leg pain, quality of life and failure of implantation (98). The combined use of interspinous spacer with decompression patients had similar results in pain and functional outcomes to the decompression alone (99,100). The meta-analysis (101) found that patients treated by interspinous spacers had high cost and high reoperation rate, and did not confer significantly more benefit to patients than decompression alone, therefore, there is no good evidence to support its use for LSS patients.
Lumbar fusion
To treat the LSS by decompression alone or decompression with fusion is an old and persistent controversy (102-106). Many kinds of lumbar fusion techniques via different approaches have been described, including posterior/posterolateral lumbar fusion, posterior lumbar interbody fusion, transforaminal lumbar interbody fusion (TLIF) and oblique lumbar interbody fusion (OLIF) (107-109) (Figure 7), and the rate of decompression plus fusion for lumbar stenosis was increased while the decompression alone was decreased (110,111).
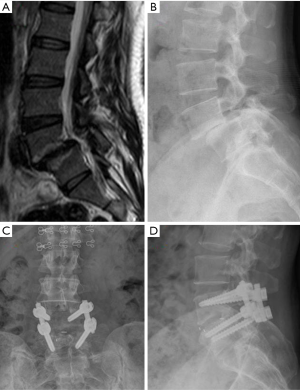
However, research suggests that decompression with fusion has small or even no benefit for most LSS patients (112,113). A recent RCT found the instrumented fusion may reduce the further progression of spondylolisthesis patients. In 2016, Försth et al. (114) published a 5 years RCT to compare the decompression plus fusion with decompression alone for LSS patients with and without spondylolisthesis, and there was no significant difference between them in clinical outcomes. Ghogawal et al. (115) published another RCT and found that for degenerative grade I spondylolisthesis patients, decompression plus fusion had statistically significant more improvement in overall physical health-related quality of life than decompression alone (score change from baseline: 14.1 vs. 7.4, P=0.02) at 4 years follow up. In both of the above two RCTs (114,115), decompression plus fusion had more blood loss, longer operative time and longer hospital stay.
Fusion is a complex operation compared with decompression alone, and therefore can potentially increase the peri-operative complications or mortality, and cost (9). Since there is a lack of evidence for advantages of fusion, this technique should be restricted to those with spinal instability, spinal deformities, or vertebral destruction caused by trauma, tumors and infections, or neuroforamen stenosis with compressed exiting nerves caused by postsurgical disk collapse (102,104).
Minimally invasive trends
Some surgeons use posterior microdecompression technique in treatment of LSS to minimize destruction to tissues with assistance by micro-endoscopy (116). Also, percutaneous endoscopic interlaminar and transforaminal decompression has been designed (117) and used in LSS (118-121).
The techniques of TLIF was also modified as minimally invasive TLIF (MI-TLIF) (122-124) and the meta-analysis found the MI-TLIF have similar clinical outcomes to the traditional TLIF technique with less trauma for LSS with or without grade I–II spondylolisthesis (125-127).
Minimally invasive techniques of lateral lumbar interbody fusion (LLIF) and OLIF (128-130) were also reported as indirect decompression choice for LSS. The efficacy and safety of indirect decompression technique was still controversial. Contrary to the direct decompression techniques, indirect decompression technique may alleviate the symptoms of radiculopathy and neurological claudication by restoration of intervertebral and foraminal heights and correction of spinal alignment (131), and is preferred for LSS patients with degenerative scoliosis by some surgeons (132). However, indirect decompression should be not performed on patients with bony lumbar stenosis, congenital stenosis and/or locked facets (133,134).
With the development of advanced image guidance systems (135), the number of surgeries performed using minimally invasive techniques has increased quickly in last decades, however, minimally invasive techniques also have some limitations, including longer learning curves, risk of some special complications (136,137), specific and limited indications, and heterogeneous clinical outcomes from different surgeons. Therefore, minimally invasive techniques in treatment of lumbar stenosis is still under development, and the safety and efficacy still need more high quality studies to prove.
Summary and key pointsOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
- LSS has high prevalence in aged population, and the most common reason for old patients to undergo the spinal surgery.
- No existence of gold standard diagnostic criteria for LSS, the diagnosis of LSS needs comprehensive consideration of the patient’s history, physical examination, radiographic images (CT or MRI), sometimes needs electromyography or nerve root block to aid diagnosis.
- Variety of non-operative options for most primary LSS patients but no evidence show which one is superior to others.
- Patients with persistent symptoms are recommended to undergo operative treatment, decompression alone or plus with fusion is an old and persistent controversy, with more evidence suggesting fusion should be used in limited indications, and interspinous spacer should be cautiously used.
- Minimally invasive technique is a new trend in spine surgery, however, their indications, safety and efficacy still need more high quality studies to prove.
AcknowledgementsOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
Funding: This work was funded by the National Natural Science Foundation of China (81501933), Zhejiang science and technology innovation project (2016R413061), Wenzhou Science and Technology Project (Y20160369). The funders had no role in the design, execution, or writing of the study.
FootnoteOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.04.13). Dr. Wu serves as an unpaid Section Editor of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- Epidemiology
- History and symptoms
- Physical examination
- Radiographic images
- Diagnosis and differential diagnosis
- Classification
- Treatment
- Summary and key points
- Acknowledgements
- Footnote
- References
- Watters WC 3rd, Baisden J, Gilbert TJ, et al. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J 2008;8:305-10. [Crossref] [PubMed]
- Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ 2016;352:h6234. [Crossref] [PubMed]
- Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med 2008;358:818-25. [Crossref] [PubMed]
- Ciol MA, Deyo RA, Howell E, et al. An assessment of surgery for spinal stenosis: time trends, geographic variations, complications, and reoperations. J Am Geriatr Soc 1996;44:285-90. [Crossref] [PubMed]
- Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J 2009;9:545-50. [Crossref] [PubMed]
- Yabuki S, Fukumori N, Takegami M, et al. Prevalence of lumbar spinal stenosis, using the diagnostic support tool, and correlated factors in Japan: a population-based study. J Orthop Sci 2013;18:893-900. [Crossref] [PubMed]
- Ishimoto Y, Yoshimura N, Muraki S, et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: the Wakayama Spine Study. Osteoarthritis Cartilage 2012;20:1103-8. [Crossref] [PubMed]
- Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30:1441-5; discussion 1446-7. [Crossref] [PubMed]
- Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010;303:1259-65. [Crossref] [PubMed]
- Amundsen T, Weber H, Lilleås F, et al. Lumbar spinal stenosis. Clinical and radiologic features. Spine (Phila Pa 1976) 1995;20:1178-86. [Crossref] [PubMed]
- Fritz JM, Delitto A, Welch WC, et al. Lumbar spinal stenosis: a review of current concepts in evaluation, management, and outcome measurements. Arch Phys Med Rehabil 1998;79:700-8. [Crossref] [PubMed]
- Lin SI, Lin RM. Disability and walking capacity in patients with lumbar spinal stenosis: association with sensorimotor function, balance, and functional performance. J Orthop Sports Phys Ther 2005;35:220-6. [Crossref] [PubMed]
- Iversen MD, Katz JN. Examination findings and self-reported walking capacity in patients with lumbar spinal stenosis. Phys Ther 2001;81:1296-306. [PubMed]
- Schönström N, Lindahl S, Willén J, et al. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res 1989;7:115-21. [Crossref] [PubMed]
- Penning L. Functional pathology of lumbar spinal stenosis. Clin Biomech (Bristol, Avon) 1992;7:3-17. [Crossref] [PubMed]
- Katz JN, Dalgas M, Stucki G, et al. Degenerative lumbar spinal stenosis. Diagnostic value of the history and physical examination. Arthritis Rheum 1995;38:1236-41. [Crossref] [PubMed]
- Suri P, Rainville J, Kalichman L, et al. Does this older adult with lower extremity pain have the clinical syndrome of lumbar spinal stenosis? JAMA 2010;304:2628-36. [Crossref] [PubMed]
- Ishimoto Y, Yoshimura N, Muraki S, et al. Association of lumbar spondylolisthesis with low back pain and symptomatic lumbar spinal stenosis in a population-based cohort: the Wakayama Spine Study. Spine (Phila Pa 1976) 2017;42:E666-E671. [PubMed]
- Norris R, Garvey T, Winter R. Why do patients seek a spine surgeon? Spine Deform 2016;4:358-64. [Crossref] [PubMed]
- Tomkins-Lane C, Melloh M, Lurie J, et al. ISSLS Prize Winner: consensus on the clinical diagnosis of lumbar spinal stenosis: results of an international Delphi study. Spine (Phila Pa 1976) 2016;41:1239-46. [Crossref] [PubMed]
- Ilkko E. Diagnosis of lumbar central spinal stenosis by plain radiography. Journal of medical imaging 1989;3:91-101.
- de Schepper EI, Overdevest GM, Suri P, et al. Diagnosis of lumbar spinal stenosis: an updated systematic review of the accuracy of diagnostic tests. Spine (Phila Pa 1976) 2013;38:E469-81. [Crossref] [PubMed]
- Cheung JP, Shigematsu H, Cheung KM. Verification of measurements of lumbar spinal dimensions in T1- and T2-weighted magnetic resonance imaging sequences. Spine J 2014;14:1476-83. [Crossref] [PubMed]
- Kim YU, Kong YG, Lee J, et al. Clinical symptoms of lumbar spinal stenosis associated with morphological parameters on magnetic resonance images. Eur Spine J 2015;24:2236-43. [Crossref] [PubMed]
- Steurer J, Roner S, Gnannt R, et al. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord 2011;12:175. [Crossref] [PubMed]
- Andreisek G, Deyo RA, Jarvik JG, et al. Consensus conference on core radiological parameters to describe lumbar stenosis—an initiative for structured reporting. Eur Radiol 2014;24:3224-32. [Crossref] [PubMed]
- Haig AJ, Geisser ME, Tong HC, et al. Electromyographic and magnetic resonance imaging to predict lumbar stenosis, low-back pain, and no back symptoms. J Bone Joint Surg Am 2007;89:358-66. [PubMed]
- Fukusaki M, Kobayashi I, Hara T, et al. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain 1998;14:148-51. [Crossref] [PubMed]
- Koc Z, Ozcakir S, Sivrioglu K, et al. Effectiveness of physical therapy and epidural steroid injections in lumbar spinal stenosis. Spine (Phila Pa 1976) 2009;34:985-9. [Crossref] [PubMed]
- Hamanishi C, Matukura N, Fujita M, et al. Cross-sectional area of the stenotic lumbar dural tube measured from the transverse views of magnetic resonance imaging. J Spinal Disord 1994;7:388-93. [Crossref] [PubMed]
- Mariconda M, Fava R, Gatto A, et al. Unilateral laminectomy for bilateral decompression of lumbar spinal stenosis: a prospective comparative study with conservatively treated patients. J Spinal Disord Tech 2002;15:39-46. [Crossref] [PubMed]
- Ullrich CG, Binet EF, Sanecki MG, et al. Quantitative assessment of the lumbar spinal canal by computed tomography. Radiology 1980;134:137-43. [Crossref] [PubMed]
- Bolender NF, Schönström NS, Spengler DM. Role of computed tomography and myelography in the diagnosis of central spinal stenosis. J Bone Joint Surg Am 1985;67:240-6. [Crossref] [PubMed]
- Lee BC, Kazam E, Newman AD. Computed tomography of the spine and spinal cord. Radiology 1978;128:95-102. [Crossref] [PubMed]
- Verbiest H. The significance and principles of computerized axial tomography in idiopathic developmental stenosis of the bony lumbar vertebral canal. Spine (Phila Pa 1976) 1979;4:369-78. [Crossref] [PubMed]
- Schonstrom NS, Bolender NF, Spengler DM. The pathomorphology of spinal stenosis as seen on CT scans of the lumbar spine. Spine (Phila Pa 1976) 1985;10:806-11. [Crossref] [PubMed]
- Schönström N, Willén J. Imaging lumbar spinal stenosis. Radiol Clin North Am 2001;39:31-53. [Crossref] [PubMed]
- Strojnik T. Measurement of the lateral recess angle as a possible alternative for evaluation of the lateral recess stenosis on a CT scan. Wien Klin Wochenschr 2001;113:53-8. [PubMed]
- Ciric I, Mikhael MA, Tarkington JA, et al. The lateral recess syndrome. A variant of spinal stenosis. J Neurosurg 1980;53:433-43. [Crossref] [PubMed]
- Mikhael MA, Ciric I, Tarkington JA, Vick NA. Neuroradiological evaluation of lateral recess syndrome. Radiology 1981;140:97-107. [Crossref] [PubMed]
- Cheung JP, Samartzis D, Shigematsu H, et al. Defining clinically relevant values for developmental spinal stenosis: a large-scale magnetic resonance imaging study. Spine (Phila Pa 1976) 2014;39:1067-76. [Crossref] [PubMed]
- Zhang L, Chen R, Liu B, et al. The nerve root sedimentation sign for differential diagnosis of lumbar spinal stenosis: a retrospective, consecutive cohort study. Eur Spine J 2016; [Epub ahead of print]. [PubMed]
- Barz T, Melloh M, Staub LP, et al. Nerve root sedimentation sign: evaluation of a new radiological sign in lumbar spinal stenosis. Spine (Phila Pa 1976) 2010;35:892-7. [Crossref] [PubMed]
- Barz T, Staub LP, Melloh M, et al. Clinical validity of the nerve root sedimentation sign in patients with suspected lumbar spinal stenosis. Spine J 2014;14:667-74. [Crossref] [PubMed]
- Macedo LG, Wang Y, Battié MC. The sedimentation sign for differential diagnosis of lumbar spinal stenosis. Spine (Phila Pa 1976) 2013;38:827-31. [Crossref] [PubMed]
- Zhang L, Chen R, Xie P, et al. Diagnostic value of the nerve root sedimentation sign, a radiological sign using magnetic resonance imaging, for detecting lumbar spinal stenosis: a meta-analysis. Skeletal Radiol 2015;44:519-27. [Crossref] [PubMed]
- Moses RA, Zhao W, Staub LP, et al. Is the sedimentation sign associated with spinal stenosis surgical treatment effect in SPORT? Spine (Phila Pa 1976) 2015;40:129-36. [Crossref] [PubMed]
- Fazal A, Yoo A, Bendo JA. Does the presence of the nerve root sedimentation sign on MRI correlate with the operative level in patients undergoing posterior lumbar decompression for lumbar stenosis? Spine J 2013;13:837-42. [Crossref] [PubMed]
- Barz C, Melloh M, Staub LP, et al. Reversibility of nerve root sedimentation sign in lumbar spinal stenosis patients after decompression surgery. Eur Spine J 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Bell GR, Rothman RH, Booth RE, et al. A study of computer-assisted tomography. II. Comparison of metrizamide myelography and computed tomography in the diagnosis of herniated lumbar disc and spinal stenosis. Spine (Phila Pa 1976) 1984;9:552-6. [Crossref] [PubMed]
- Jia LS, Shi ZR. MRI and myelography in the diagnosis of lumbar canal stenosis and disc herniation. A comparative study. Chin Med J (Engl) 1991;104:303-6. [PubMed]
- Bischoff RJ, Rodriguez RP, Gupta K, et al. A comparison of computed tomography-myelography, magnetic resonance imaging, and myelography in the diagnosis of herniated nucleus pulposus and spinal stenosis. J Spinal Disord 1993;6:289-95. [Crossref] [PubMed]
- Jacobson RE. Lumbar stenosis. An electromyographic evaluation. Clin Orthop Relat Res 1976;68-71. [PubMed]
- Plastaras CT. Electrodiagnostic challenges in the evaluation of lumbar spinal stenosis. Phys Med Rehabil Clin N Am 2003;14:57-69. [Crossref] [PubMed]
- Yagci I, Gunduz OH, Ekinci G, et al. The utility of lumbar paraspinal mapping in the diagnosis of lumbar spinal stenosis. Am J Phys Med Rehabil 2009;88:843-51. [Crossref] [PubMed]
- Zhang GL, Zhen P, Chen KM, et al. Application of selective nerve root blocks in limited operation of the lumbar spine. Zhongguo Gu Shang 2014;27:601-4. [PubMed]
- Kikuchi S. Anatomical and experimental studies of nerve root infiltration. Nihon Seikeigeka Gakkai Zasshi 1982;56:605-14. [PubMed]
- Han TR, Paik NJ, Lee SJ, et al. A new method to measure caudal motor conduction time using magnetic stimulation. Muscle Nerve 2004;30:727-31. [Crossref] [PubMed]
- Shen N, Wang G, Chen J, et al. Evaluation of degree of nerve root injury by dermatomal somatosensory evoked potential following lumbar spinal stenosis. Neural Regen Res 2008;3:1249-52.
- Storm SA, Kraft GH. The clinical use of dermatomal somatosensory evoked potentials in lumbosacral spinal stenosis. Phys Med Rehabil Clin N Am 2004;15:107-15. [Crossref] [PubMed]
- Genevay S, Atlas SJ, Katz JN. Variation in eligibility criteria from studies of radiculopathy due to a herniated disc and of neurogenic claudication due to lumbar spinal stenosis: a structured literature review. Spine (Phila Pa 1976) 2010;35:803-11. [Crossref] [PubMed]
- Nadeau M, Rosas-Arellano MP, Gurr KR, et al. The reliability of differentiating neurogenic claudication from vascular claudication based on symptomatic presentation. Can J Surg 2013;56:372-7. [Crossref] [PubMed]
- Ogbera AO, Adeleye O, Solagberu B, et al. Screening for peripheral neuropathy and peripheral arterial disease in persons with diabetes mellitus in a Nigerian University Teaching Hospital. BMC Res Notes 2015;8:533. [Crossref] [PubMed]
- Haig AJ, Park P, Henke PK, et al. Reliability of the clinical examination in the diagnosis of neurogenic versus vascular claudication. Spine J 2013;13:1826-34. [Crossref] [PubMed]
- Adamova B, Vohanka S, Dusek L. Differential diagnostics in patients with mild lumbar spinal stenosis: the contributions and limits of various tests. Eur Spine J 2003;12:190-6. [PubMed]
- Arnoldi CC, Brodsky AE, Cauchoix J, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res 1976;4-5. [PubMed]
- Ammendolia C, Stuber KJ, Rok E, et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev 2013;CD010712. [PubMed]
- Ammendolia C, Stuber K, de Bruin LK, et al. Nonoperative treatment of lumbar spinal stenosis with neurogenic claudication: a systematic review. Spine (Phila Pa 1976) 2012;37:E609-16. [Crossref] [PubMed]
- Deyo RA, Bass JE. Lifestyle and low-back pain. The influence of smoking and obesity. Spine (Phila Pa 1976) 1989;14:501-6. [Crossref] [PubMed]
- Tomkins-Lane CC, Lafave LM, Parnell JA, et al. The spinal stenosis pedometer and nutrition lifestyle intervention (SSPANLI): development and pilot. Spine J 2015;15:577-86. [Crossref] [PubMed]
- Wong JJ, Côté P, Ameis A, et al. Are non-steroidal anti-inflammatory drugs effective for the management of neck pain and associated disorders, whiplash-associated disorders, or non-specific low back pain? A systematic review of systematic reviews by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Eur Spine J 2016;25:34-61. [Crossref] [PubMed]
- Roelofs PD, Deyo RA, Koes BW, et al. Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst Rev 2008;CD000396. [PubMed]
- Coronado-Zarco R, Cruz-Medina E, Arellano-Hernández A, et al. Effectiveness of calcitonin in intermittent claudication treatment of patients with lumbar spinal stenosis: a systematic review. Spine (Phila Pa 1976) 2009;34:E818-22. [Crossref] [PubMed]
- Peng K, Chen L, Peng J, et al. Effects of calcitonin on lumbar spinal stenosis: a systematic review and meta-analysis. Int J Clin Exp Med 2015;8:2536-44. [PubMed]
- Krebs EE, Lurie JD, Fanciullo G, et al. Predictors of long-term opioid use among patients with painful lumbar spine conditions. J Pain 2010;11:44-52. [Crossref] [PubMed]
- Tomkins CC, Dimoff KH, Forman HS, et al. Physical therapy treatment options for lumbar spinal stenosis. J Back Musculoskelet Rehabil 2010;23:31-7. [Crossref] [PubMed]
- Macedo LG, Hum A, Kuleba L, et al. Physical therapy interventions for degenerative lumbar spinal stenosis: a systematic review. Phys Ther 2013;93:1646-60. [Crossref] [PubMed]
- Jordan SE. Assessment: use of epidural steroid injections to treat radicular lumbosacral pain: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007;69:1191. [Crossref] [PubMed]
- Chou R, Hashimoto R, Friedly J, et al. Epidural Corticosteroid Injections for Radiculopathy and Spinal Stenosis: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:373-81. [Crossref] [PubMed]
- Armon C, Evans RWTherapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Addendum to assessment: prevention of post-lumbar puncture headaches: report of the therapeutics and technology assessment subcommittee of the american academy of Neurology. Neurology 2005;65:510-2. [Crossref] [PubMed]
- Friedly JL, Comstock BA, Turner JA, et al. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med 2014;371:11-21. [Crossref] [PubMed]
- Hong SW, Choi KY, Ahn Y, et al. A comparison of unilateral and bilateral laminotomies for decompression of L4-L5 spinal stenosis. Spine (Phila Pa 1976) 2011;36:E172-8. [Crossref] [PubMed]
- Gelalis ID, Stafilas KS, Korompilias AV, et al. Decompressive surgery for degenerative lumbar spinal stenosis: long-term results. Int Orthop 2006;30:59-63. [Crossref] [PubMed]
- Overdevest G, Vleggeert-Lankamp C, Jacobs W, et al. Effectiveness of posterior decompression techniques compared with conventional laminectomy for lumbar stenosis. Eur Spine J 2015;24:2244-63. [Crossref] [PubMed]
- Kanbara S, Yukawa Y, Ito K, et al. Surgical outcomes of modified lumbar spinous process-splitting laminectomy for lumbar spinal stenosis. J Neurosurg Spine 2015;22:353-7. [Crossref] [PubMed]
- Baghdadi YM, Moussallem CD, Shuaib MA, et al. Lumbar spinous process-splitting laminoplasty: a novel technique for minimally invasive lumbar decompression. Orthopedics 2016;39:e950-6. [Crossref] [PubMed]
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008;358:794-810. [Crossref] [PubMed]
- Pearson A, Blood E, Lurie J, et al. Predominant leg pain is associated with better surgical outcomes in degenerative spondylolisthesis and spinal stenosis: results from the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976) 2011;36:219-29. [Crossref] [PubMed]
- Amundsen T, Weber H, Nordal H, et al. Lumbar spinal stenosis: conservative or surgical management?: A prospective 10-year study. Spine (Phila Pa 1976) 2000;25:1424-35; discussion 1435-6. [Crossref] [PubMed]
- Malmivaara A, Slätis P, Heliövaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine (Phila Pa 1976) 2007;32:1-8. [Crossref] [PubMed]
- Siddiqui M, Smith FW, Wardlaw D. One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine (Phila Pa 1976) 2007;32:1345-8. [Crossref] [PubMed]
- Sobottke R, Schlüter-Brust K, Kaulhausen T, et al. Interspinous implants (X Stop, Wallis, Diam) for the treatment of LSS: is there a correlation between radiological parameters and clinical outcome? Eur Spine J 2009;18:1494-503. [Crossref] [PubMed]
- Park SC, Yoon SH, Hong YP, et al. Minimum 2-year follow-up result of degenerative spinal stenosis treated with interspinous u (coflex). J Korean Neurosurg Soc 2009;46:292-9. [Crossref] [PubMed]
- Richards JC, Majumdar S, Lindsey DP, et al. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine (Phila Pa 1976) 2005;30:744-9. [Crossref] [PubMed]
- Nunley PD, Shamie AN, Blumenthal SL, et al. Interspinous process decompression: expanding treatment options for lumbar spinal stenosis. Biomed Res Int 2016;2016:3267307.
- Moojen WA, Arts MP, Jacobs WC, et al. Interspinous process device versus standard conventional surgical decompression for lumbar spinal stenosis: randomized controlled trial. BMJ 2013;347:f6415. [Crossref] [PubMed]
- Puzzilli F, Gazzeri R, Galarza M, et al. Interspinous spacer decompression (X-STOP) for lumbar spinal stenosis and degenerative disk disease: a multicenter study with a minimum 3-year follow-up. Clin Neurol Neurosurg 2014;124:166-74. [Crossref] [PubMed]
- Beyer F, Yagdiran A, Neu P, et al. Percutaneous interspinous spacer versus open decompression: a 2-year follow-up of clinical outcome and quality of life. Eur Spine J 2013;22:2015-21. [Crossref] [PubMed]
- Kim KA, McDonald M, Pik JH, et al. Dynamic intraspinous spacer technology for posterior stabilization: case-control study on the safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. Neurosurg Focus 2007;22:E7. [Crossref] [PubMed]
- Richter A, Halm HF, Hauck M, et al. Two-year follow-up after decompressive surgery with and without implantation of an interspinous device for lumbar spinal stenosis: a prospective controlled study. J Spinal Disord Tech 2014;27:336-41. [Crossref] [PubMed]
- Wu AM, Zhou Y, Li QL, et al. Interspinous spacer versus traditional decompressive surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PLoS One 2014;9:e97142. [Crossref] [PubMed]
- Wu AM, Tong TJ, Wang XY. A rethink of fusion surgery for lumbar spinal stenosis. J Evid Based Med 2016;9:166-9. [Crossref] [PubMed]
- Tye EY, Anderson J, Haas A, et al. Decompression vs. decompression and fusion for degenerative lumbar stenosis (DLS) in a workers' compensation setting. Spine (Phila Pa 1976) 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Peul WC, Moojen WA. Fusion for lumbar spinal stenosis—safeguard or superfluous surgical implant? N Engl J Med 2016;374:1478-9. [Crossref] [PubMed]
- Omidi-Kashani F, Hasankhani EG, Ashjazadeh A. Lumbar spinal stenosis: who should be fused? An updated review. Asian Spine J 2014;8:521-30. [Crossref] [PubMed]
- Greenway FE, Papadopoulos MC. Fusion surgery for lumbar spinal stenosis? J Spine Surg 2016;2:154-7. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Talia AJ, Wong ML, Lau HC, et al. Comparison of the different surgical approaches for lumbar interbody fusion. J Clin Neurosci 2015;22:243-51. [Crossref] [PubMed]
- Liu X, Wang Y, Qiu G, et al. A systematic review with meta-analysis of posterior interbody fusion versus posterolateral fusion in lumbar spondylolisthesis. Eur Spine J 2014;23:43-56. [Crossref] [PubMed]
- Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine (Phila Pa 1976) 2013;38:916-26. [Crossref] [PubMed]
- Jancuska JM, Hutzler L, Protopsaltis TS, et al. Utilization of lumbar spinal fusion in New York State: trends and disparities. Spine (Phila Pa 1976) 2016;41:1508-14. [Crossref] [PubMed]
- Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am 1995;77:1036-41. [Crossref] [PubMed]
- Hallett A, Huntley JS, Gibson JN. Foraminal stenosis and single-level degenerative disc disease: a randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine (Phila Pa 1976) 2007;32:1375-80. [Crossref] [PubMed]
- Försth P, Ólafsson G, Carlsson T, et al. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N Engl J Med 2016;374:1413-23. [Crossref] [PubMed]
- Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus Fusion versus Laminectomy Alone for Lumbar Spondylolisthesis. N Engl J Med 2016;374:1424-34. [Crossref] [PubMed]
- Weiner BK, Walker M, Brower RS, et al. Microdecompression for lumbar spinal canal stenosis. Spine (Phila Pa 1976) 1999;24:2268-72. [Crossref] [PubMed]
- Jasper GP, Francisco GM, Telfeian AE. Transforaminal endoscopic discectomy with foraminoplasty for the treatment of spondylolisthesis. Pain Physician 2014;17:E703-8. [PubMed]
- Kambin P, Casey K, O'Brien E, et al. Transforaminal arthroscopic decompression of lateral recess stenosis. J Neurosurg 1996;84:462-7. [Crossref] [PubMed]
- Chiu JC. Evolving transforaminal endoscopic microdecompression for herniated lumbar discs and spinal stenosis. Surg Technol Int 2004;13:276-86. [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:931-9. [Crossref] [PubMed]
- Nellensteijn J, Ostelo R, Bartels R, et al. Transforaminal endoscopic surgery for lumbar stenosis: a systematic review. Eur Spine J 2010;19:879-86. [Crossref] [PubMed]
- Luo P, Shao RX, Wu AM, et al. Transforaminal lumbar interbody fusion with unilateral pedicle screw and contralateral percutaneous transfacet screw fixation for the treatment of lumbar degenerative disorders. Turk Neurosurg 2016;26:763-70. [PubMed]
- Kim CW, Doerr TM, Luna IY, et al. Minimally invasive transforaminal lumbar interbody fusion using expandable technology: a clinical and radiographic analysis of 50 patients. World Neurosurg 2016;90:228-35. [Crossref] [PubMed]
- Elboghdady IM, Naqvi A, Jorgenson AY, et al. Minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis. Ann Transl Med 2014;2:99. [PubMed]
- Wu AM, Chen CH, Shen ZH, et al. The outcomes of minimally invasive versus open posterior approach spinal fusion in treatment of lumbar spondylolisthesis: the current evidence from prospective comparative studies. Biomed Res Int 2017;2017:8423638.
- Sidhu GS, Henkelman E, Vaccaro AR, et al. Minimally invasive versus open posterior lumbar interbody fusion: a systematic review. Clin Orthop Relat Res 2014;472:1792-9. [Crossref] [PubMed]
- Goldstein CL, Macwan K, Sundararajan K, et al. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine 2016;24:416-27. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J 2017;26:671-8. [Crossref] [PubMed]
- McAfee PC, Regan JJ, Geis WP, et al. Minimally invasive anterior retroperitoneal approach to the lumbar spine. Emphasis on the lateral BAK. Spine (Phila Pa 1976) 1998;23:1476-84. [Crossref] [PubMed]
- Fujibayashi S, Hynes RA, Otsuki B, et al. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine (Phila Pa 1976) 2015;40:E175-82. [Crossref] [PubMed]
- Pereira EA, Farwana M, Lam KS. Extreme lateral interbody fusion relieves symptoms of spinal stenosis and low-grade spondylolisthesis by indirect decompression in complex patients. J Clin Neurosci 2017;35:56-61. [Crossref] [PubMed]
- Malham GM, Parker RM, Goss B, et al. Clinical results and limitations of indirect decompression in spinal stenosis with laterally implanted interbody cages: results from a prospective cohort study. Eur Spine J 2015;24:339-45. [Crossref] [PubMed]
- Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35:S331-7. [Crossref] [PubMed]
- Bourgeois AC, Faulkner AR, Pasciak AS, et al. The evolution of image-guided lumbosacral spine surgery. Ann Transl Med 2015;3:69. [PubMed]
- Sclafani JA, Kim CW. Complications associated with the initial learning curve of minimally invasive spine surgery: a systematic review. Clin Orthop Relat Res 2014;472:1711-7. [Crossref] [PubMed]
- Chaichankul C, Poopitaya S, Tassanawipas W. The effect of learning curve on the results of percutaneous transforaminal endoscopic lumbar discectomy. J Med Assoc Thai 2012;95:S206-12. [PubMed]
Cite this article as: Wu AM, Zou F, Cao Y, Xia DD, He W, Zhu B, Chen D, Ni WF, Wang XY, Kwan K. Lumbar spinal stenosis: an update on the epidemiology, diagnosis and treatment. AME Med J 2017;2:63.