
Stereotactic body radiation therapy versus surgery for patients with stage I non-small cell lung cancer
Introduction -What is SBRT?
Although the incidence rate of lung cancer has decreased over the last decade, its mortality rate remains the highest among all cancer-related deaths worldwide (1,2). Early detection, for example by CT examination, is very important to combat the high mortality rate due to lung cancer. Although lobectomy is the treatment choice for patients with early stage non-small cell lung cancer (NSCLC) (3,4), relatively few patients are candidates for lobectomy, as many are elderly or have poor pulmonary function. These patients are frequently treated with sublobar resection (SLR) or stereotactic body radiotherapy (SBRT), both of which have been shown to be effective and safe. SBRT, which delivers high radiation doses to focal lung tumors, has particularly helped to avoid radiation-induced damage to normal lungs (Figure 1). Treatment frequency is delivered via hypofractionated schedules, consisting of 3–5 fractions of 10- 15Gy per fraction. Toxicity may also be reduced by recent advanced technologies, including tumor-tracking, respiratory gating, image-guided radiotherapy systems.
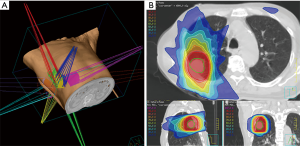
This review summarizes comparisons of SBRT with surgery, including lobectomy and SLR, for patients with stage I NSCLC.
Current status of SBRT for patients with stage I NSCLC
SBRT for patients with medically inoperable stage I NSCLC
SBRT is widely used to treat patients with medically inoperable and peripherally located early stage NSCLC, having shown efficacy and safety in these patients (5-9). For example, a Japanese phase II trial, the Japan Clinical Oncology Group (JCOG) study 0403, reported that the 3-year overall survival (OS) and local control rates of SBRT (48 Gy in four fractions) for patients with c-stage IA medically inoperative NSCLC were 59.9% and 88%, respectively, with the rate of grade ≥4 toxicities being only 1% (8). The RTOG0236, phase II trial for patients with c-stage IA or IB medically inoperative NSCLC, found that a higher dose/fraction schedule for SBRT, such as 54 Gy in 3 fractions, resulted in a 3-year OS rate of 55.0%, similar to that in JCOG0403. However, the local control rate, 97.6%, was higher than that of JCOG0403, likely because of its higher dose/fraction schedule. The rate of grade ≥4 toxicities was 3.6% (7). The findings of several studies suggested that SBRT is effective and safe for patients with medically inoperable early NSCLC (Table 1). Moreover, the guidelines of the National Comprehensive Cancer Network (NCCN), as well as Japanese guidelines, have recommended SBRT as standard treatment for these patients (3,4).
Table 1
| Author (year) | Stage (stage IB) | Study design | N | Dose/fraction (BED10)# | Prescription method | 3-year overall survival | 3-year local control | Toxicity grade 3≥ (Grade 4≥) |
|---|---|---|---|---|---|---|---|---|
| Baumann P (2009) (5) | IA/B | Phase II | 57 | 45 Gy/3 fr (112.5 Gy) | PTV marginal | 60% | 92% | 29.9% |
| 30.0% | 1.8% | |||||||
| Fakiris AJ (2009) (6) | IA/B | Phase II | 70 | IA: 60 Gy/3 fr (180 Gy), IB: 66 Gy/3 fr | PTV marginal | 42.7% | N.A.* | 17.1% |
| 51.4% | IB: 66 Gy/3 fr (192 Gy) | 8.6% | ||||||
| RTOG0236 (2010) (7) | IA/B | Phase II | 55 | 54 Gy/3 fr (151.2 Gy) | PTV marginal | 55%, (5 y 40%) | 97.6%, (5 y 93%) | 16.3% |
| 20.0% | 3.6% | |||||||
| JCOG0403 (2015) (8) | IA | Phase II | 100 | 48 Gy/4 fr (105.6 Gy) | Isocenter | 59.9% | 88% | 10.6% |
| 1% | ||||||||
| RTOG0915 (9) | IA/B | Randomized Phase II | 45 | 48 Gy/4 fr (105.6 Gy) | PTV marginal | 77.7% (2 y) | 92.7% (1 y) | 13.3% |
| 11.1% | NA* | |||||||
| RTOG0915 (9) | IA/B | Randomized Phase II | 39 | 34 Gy/1 fr (150 Gy) | PTV marginal | 61.3% (2 y) | 97% (1 y) | 10.3% |
| 18.0% | NA* |
*NA, not available, #BED10, biologically effective dose based on α/β =10 Gy.
In contrast, the optimal dose/fraction schedule remains unclear. A Japanese multi-institutional cohort study suggested that the optimal dose/fraction schedule be based on the biologically effective dose (BED) (10). For example, the effects of various dose/fraction schedules, consisting of different fraction sizes and total doses, were assessed using the BED in a linear-quadratic model (11). In that study, the BED was defined as nd(1 +d/α/β), with gray units, where n is the fractionation number, d is the daily dose, and α/β is assumed to be 10 for tumors. The authors of that study concluded that local control and survival rates were better with a BED of ≥100 Gy than of <100 Gy for all dose/fraction schedules. A meta-analysis of 34 observational studies containing a total 2,587 patients also assessed the optimal BED range for treatment of c-Stage I NSCLC (12). That study found that Grade 3–5 adverse events were more frequent in patients with high BED (>146 Gy), suggesting that SBRT using a medium (83.2–106 Gy), or medium-to-high (106–146 Gy) BED may be more beneficial than SBRT using a low (<83.2 Gy) or high (>146 Gy) BED. To determine the optimal dose/fraction schedule, we started a randomized phase III study (JCOG1408) in February 2016 comparing 42 Gy in four fractions (BED10: 86.1 Gy) with 55 Gy in four fractions (BED10: 130.6 Gy) for patients with medically inoperable stage IA NSCLC and small lung lesions clinically diagnosed as primary lung cancer (13). The standard arm, 42 Gy in four fractions prescribed at the D95% of the planning target volume, which is considered equal to 48 Gy in four fractions at the isocenter using an old dose calculation algorithm, is the standard treatment in Japan for medically inoperable stage IA NSCLC.
SBRT for patients with medically operable stage I NSCLC
Compared with the number of publications evaluating SBRT for medically inoperable early NSCLC, fewer have assessed SBRT for medically operable early NSCLC (8,14-16). A retrospective study evaluation SBRT for 87 patients with medically operable stage I NSCLC using a Japanese multi-institutional database studied several dose/fraction schedules, involving 45–72.5 Gy in 3–10 fractions (median BED10 116 Gy; range, 100–141 Gy) (14). That study reported that 5-year OS and local control rates were 72% and 62%, respectively, in the Stage IA subgroup and 92% and 73%, respectively, in the Stage IB subgroup, with no severe toxicities. In the phase II JCOG0403 trial, involving 48 Gy in 4 fractions, the 3-year OS and local control rates were 76.0% and 88%, respectively (6). In another phase II study, RTOG0618, involving 54 Gy in 3 fractions, the 2-year OS and local control rates were 84.4% and 92.3%, respectively (16). No patients in these two phase II trials experienced grade ≥ 4 toxicity. Table 2 summarizes treatment results for patients with medically operable NSCLC, showing that the survival rates after SBRT and surgery were comparable. However, because of the lack of phase III trials comparing surgery with SBRT, guidelines continue to recommend surgery, especially lobectomy, as standard treatment for medically operable patients (8,9).
Table 2
| Author (year) | Stage (stage IB) | Study design | N | Dose/fraction (BED10)# | Prescription method | 3-year overall survival | 3-year local control | Toxicity grade 3≥ (Grade 4≥) |
|---|---|---|---|---|---|---|---|---|
| Onish H (2011) (14) | IA/B | retrospective | 87 | 45–72.5 Gy/3–10 fr (100–144 Gy) | Isocenter | IA: 72% (5y) | IA: 92% (5 y) | 9.2% |
| 25.3% | IB: 62% (5y) | IB: 73% (5 y) | 0% | |||||
| Lagerwaard FJ (2012) (15) | IA/B | retrospective | 177 | 60 Gy/3– 8 fr (105–180 Gy) | PTV marginal | 84.7% | 93% | 5% |
| 40.1% | NA* | |||||||
| JCOG0403 (2015) (8) | IA | Phase II | 64 | 48 Gy/4 fr (105.6 Gy) | Isocenter | 76% | 86% | 6.2% |
| 0% | ||||||||
| RTOG0618 (2013) (16) | IA/B | Phase II | 26 | 54 Gy/3 fr (151.2 Gy) | PTV marginal | 84.4% | 92.3% | 15.4% |
| 11.5% | (2 y) | (2 y) | 0% |
*NA, not available; #BED10, biologically effective dose based on α/β =10 Gy.
SBRT versus surgery for patients with stage I NSCLC
SBRT versus lobectomy for patients with medically operable stage I NSCLC
SBRT for patients with medically operable stage I NSCLC was found to result in a local control rate >90% and a 3-years OS rate of 70- 80% (Table 2). However, because mediastinal lymph node dissection or sampling is usually performed during lobectomy, concerns remain about the risk of local or nodal recurrence after SBRT, either of which could lead to poorer OS than after lobectomy. SBRT and lobectomy having been compared using propensity score-matching (PSM) analyses (Table 3) (17-23). A survey of the Surveillance, Epidemiology, and End Results (SEER) database from 2003 to 2009 assessed, OS in a large number of patients with early-stage, node-negative NSCLC who underwent lobectomy (n=7,215), or SBRT (n=382) (19). Prior to maching, SBRT was associated with a lower risk of death [hazard ratio (HR), 0.45; 95% confidence interval (CI), 0.27–0.75; P<0.001] during the 6 months, but a higher risk of death (HR, 1.66; 95% CI, 1.39–1.99; P<0.001) after 6 months. After PSM, resulting in 251 well-matched pairs, the two modalities were associated with a similar risk of OS (HR, 1.01; 95% CI: 0.74–1.38; P=0.94). These findings suggested that lobectomy was the optimal treatment for older individuals able to undergo surgery. However, SBRT was promising for frail patients and those of advanced age because of a lower risk of periprocedural mortality and encouraging long-term survival. Another study, 64 matched pairs found 3-year OS rates were similar in patients who underwent lobectomy and SBRT (77% vs. 80%, P=0.803) (23). A study of 73 matched pairs reported, although 3-year OS rates tended to favor surgery, 5-year OS rates were similar in patients who underwent lobectomy and SBRT (80% vs. 53%, P= 0.082) (20).
Table 3
| Author (year) | Data source | N | Surgery | SBRT | OS (unmatched) | OS (matched) | Conclusions | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | SBRT | Surgery | SBRT | |||||||
| Verstegen NE (2013) (17) | c-I/II | Surgery: 86 SBRT: 527 Matched: each 64 |
Lobectomy | 60 Gy/5 fr (BED10 ≥100 Gy) | - | 77% (3 y) | 80% (3 y) | OS rates did not differ significantly | ||
| P= 0.803 | ||||||||||
| Shirvani SM (2014) (18) | c-IA/IB SEER database (2003- 2009) | Surgery: 7,215 SBRT: 382 Matched: each 251 |
Lobectomy | Several dose/fraction schedule BED10 ≥100 Gy) | Lobectomy vs. SBRT (after initial 6 months): HR 1.66 (95% CI: 1.39–1.99), P<0.001 | Lobectomy vs. SBRT: HR 1.01 (95% CI: 0.74-1.38), P= 0.94 | Lobectomy is optional for older adults able to undergo surgery. SBRT is promising for frail patients and those of advanced age because of a lower risk of periprocedural mortality and encouraging long-term survival | |||
| Eba J (2014) (19) | c-IA JCOG0203/0403 database | Surgery: 219 SBRT: 40 Matched: each 21 |
Lobectomy | 48 Gy/4 fr | 87.7% (5 y) | 67.0% (5 y) | HR: 9.00 (1.14–71.04), P=0.037 | The point estimates of HR for OS favored lobectomy over SBRT in a limited number of patients. Randomized controlled trials are needed for a valid comparison to evaluate the non-inferiority of SBRT | ||
| HR 1.51, P= 0.28 | ||||||||||
| Mokhles S (2015) (20) | c-IA/B | Surgery: 96S BRT: 481 Matched: each 73 |
Lobectomy | 54–60 Gy/3–8 fr | – | 80% (5 y) | 53% (5 y) | OS rates did not differ significantly in propensity matched patients with stage I NSCLC treated surgically or with SBRT | ||
| P=0.082 | ||||||||||
| Chang JY (2015) (21) | c-IA/IB STARS + ROSEL database | Surgery: 27 SBRT: 31 |
Lobectomy | 54 Gy/3 fr | – | 79% (3 y) | 95% (3 y) | SBRT may be an option for treating operable stage I NSCLC | ||
| P=0.037 | ||||||||||
| Hamaji M (2015) (22) | c-IA/B | Surgery: 412 SBRT: 104 Matched: each 41 |
Lobectomy | Median: 48 Gy/4 fr | 78.3% (5 y) | 40.6% (5 y) | 68.5% (5 y) | 37.3% (5 y) | VATS lobectomy may offer significantly more favorable long-term outcomes than SBRT in potentially operable patients with biopsy proven clinical stage I NSCLC | |
| P< 0.0001 | P=0.0016 | |||||||||
| Rosen JE (2016) (23) | c-IA/B National Cancer database | Surgery: 13,562 SBRT: 1,781 (operable) Matched: 1,781 |
Lobectomy | Variable | - | 59% (5 y) | 29% (5 y) | Among healthy patients with clinical stage I NSCLC, lobectomy is associated with a significantly better outcome than SBRT | ||
| P<0.001 | ||||||||||
These studies from western countries suggested that OS rates were similar in patients who underwent SBRT and lobectomy. Although STARS trial (Randomized study to compare CyberKnife to surgical resection in stage I non-small cell lung cancer: NCT00840749) was initiated in 2009, and the ROSEL trial (Trial of either surgery or stereotactic radiotherapy for early stage IA lung cancer: NCT00687986) was initiated in 2008, these two randomized phase III trials had to be closed because of slow accrual. A pooled analysis of the 58 patients enrolled in these two trials and randomized to SBRT (n=31) and lobectomy (n=27) found that these two groups had 3-year OS rates of 95% (95% CI, 85–100%) and 79% (95% CI: 64–97%), respectively (HR, 0.14; 95% CI, 0.017–1.190; log-rank P= 0.037) (21). Although these results were encouraging, there were several limitations, such as the small number of patients, the study not being a true randomized phase III trial, and the poorer results of lobectomy (3-year OS: 79%) compared with studies in Japan, such as JCOG0201, which reported a 5-year OS rate of 90.6% (24).
Two Japanese reports found that OS rates after PSM were significantly better for lobectomy than for SBRT (19,22). These differences were thought to be due to the lower mortality rate after lobectomy and the higher cause-specific survival (CSS) rate in the lobectomy group. Regional lymph node control was found to be similar in patients who underwent lobectomy and SBRT (19), suggesting that routine systematic mediastinal LN dissection did not have a therapeutic effect, but rather identified candidates for adjuvant chemotherapy, which may be associated with a significant difference in distant control when compared with SBRT.
SBRT versus sublobar resection for patients with stage I NSCLC at high risk for lobectomy
Theoretically, due to the omission of mediastinal lymph node dissection or sampling, SLR may be similar to SBRT as a local treatment modality for patients at high risk for lobectomy. According to NCCN guidelines, SBRT is also an appropriate option for patients at high surgical risk who are able to tolerate SLR but not lobectomy, such as aged >75 years and those with poor lung function (8). PSM analyses have compared SBRT and SLR (Table 4) (25-34). A survey of the SEER database, comparing SLR and SBRT in 112 pairs of PSM patients in from 2001 to 2007, found that these two modalities were associated with similar OS rates (HR, 0.82; 95% CI: 0.45–1.12; P=0.38) (27). A comparison of SLR and SBRT, based on a median age of 76 years, a performance status of 0–1, a median tumor diameter < 20 mm, a median 1 second forced expiratory volume (FEV1) of <1.8 L and a median Charlson comorbidity index of 1, found that, before PSM, the 5-year OS rate was higher in the SLR than in the SBRT group (60.5% vs. 40.3%, P=0.008) but 5-year CSS rates were similar (26.3% vs. 33.8%, P=0.215). A study of 53 matched pairs found that patient series, 5-year OS (55.6% vs. 40.4%, P=0.124) and CSS (30.3% vs. 35.3%, P=0.427) rates were similar in the SLR and SBRT groups (29). Most of the studies cited in Table 4 showed no significant differences in OS and CSS between SBRT and SLR after PSM. These similar outcomes may have been due to the inclusion of both groups of patients with comorbidities and to both modalities being local treatment. Prospective trials will likely show that OS and CSS are similar in patients undergoing SBRT and SLR, suggesting that SBRT may be an alternative to SLR in high-risk patients who cannot tolerate lobectomy because of medical comorbidities.
Table 4
| Author (year) | Data Source | N | Surgery | SBRT | OS (unmatched) | OS (matched) | Conclusions | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | SBRT | Surgery | SBRT | |||||||
| Palma D (2011) (25) | c-IA/B 17 centers in Netherland | Surgery: 109SBRT: 81Matched: each 60 | Lobectomy: 82%Sublobar: 15%Pneumonectomy: 3% | 60 Gy/5 fr | -P=0.22 | 60% (3 y) | 42% (3 y) | Similar OS outcomes are achieved with surgery or SBRT for stage I NSCLC in elderly patients | ||
| Shirvani SM (2012) (26) | c-IA/IB SEER database (2001- 2007) | Surgery: 1,278SBRT: 124Matched: each 112 | Sublobar | Several dose/fraction schedule (BED10 ≥100 Gy) | - | Sublobar vs. SBRT: HR 0.82 (95% CI: 0.45-1.12), P= 0.38 | Survival after SBRT was similar to that after sublobar resection | |||
| Varlotto J (2013) (27) | c-IA/IB | Surgery: 180SBRT: 137Matched: each 72 (lobectomy)Matched: each 17 (sublobar) | Lobectomy: 132 Sublobar: 48 | 60 Gy/3 fr (BED10 ≥100 Gy) | P=0.00486.3% (5 y: sublobar)31.7% (5 y)P=0.003 | 69.2% (5 y: lobectomy) | 33.7% (5 y) | OS was superior in SLR compared with SBRT matched pairs base.However, a multivariate analysis that included propensity scores as a covariate revealed that the hazard ratio for OS was not significant | ||
| Crabtree TD (2014) (28) | c-IA/B | Surgery: 458SBRT: 151Matched: each 56 | Lobectomy: 78%Sublobar: 18%Pneumonectomy: 4% | 54 Gy/3 fr (BED10 ≥100 Gy) | 78% (3 y) | 47% (3 y) | 60% (3 y) | 52% (3 y) | Although surgical resection seems to result in better OS and DFS versus SBRT, matching these disparate cohorts of patients remains challenging | |
| P= 0.05 | ||||||||||
| Matsuo Y (2014) (29) | c-IA/IB | Surgery: 65SBRT: 115Matched: each 53 | Sublobar | 48 or 56 Gy/4 fr60 Gy/8 fr (central) | 60.5% (5 y) | 40.3% (5 y) | 55.6% (5 y) | 40.4% (5 y) | SBRT can be an alternative treatment option to SLR for patients who cannot tolerate lobectomy | |
| P= 0.008 | P= 0.124 | |||||||||
| Zheng X (2014) (30) | c-IA/IB meta-analysis | Surgery: 7,071SBRT: 4,850Matched: operability, <70 y.o. | Lobectomy: 86.2% Sublobar 13.8% | Several dose/fraction schedules (BED10 ≥100 Gy) | 66.1% | 41.2% | 68% | 82% | After adjustment for these differences, OS do not differ significantly between SBRT and surgery in patients with operable stage I NSCLC | |
| (5y: lobectomy) | (5y) | (5y: lobectomy) | (5y) | |||||||
| 71.7% | 66% | |||||||||
| (5y: sublobar) | (5y: sublobar) | |||||||||
| Port JL (2014) (31) | c-IA | Surgery: 76SBRT: 23 | Sublobar (Wedge ± Brachy) | Median: 48 Gy/4 fr | P= 0.357 | 87% (3 y) | 75% (3 y) | Patients with clinical stage IA NSCLC treated by SBRT appear to have higher overall disease recurrence than those treated by SLR. However, there was no significant difference in DFS | ||
| Puri V (2015) (32) | c-IA/B | Surgery:111,731SBRT: 5,887Matched: each 5355 | Lobectomy: 71.4%Sublobar: 27.4%Pneumonectomy: 1.2% | Median: 54 Gy | 61.7% (3 y: sublobar) 46% (3 y) |
68% (3 y) | 47% (3 y) | Patients selected for surgery have improved survival compared with SBRT | ||
| Matched: each 4555 (sublobar) | ||||||||||
| Ma L (2016) (33) | Meta-analysis | Surgery: 3,436 | Variable | Variable | 82.8% (3 y) | 6% (3 y) | 84% (3 y) | 87% (3 y) | After adjusting for these differences, OS and DFS did not differ significantly between the two techniques | |
| SBRT: 4,433 | ||||||||||
| Matched: age, operability | Significant difference | No significant difference | ||||||||
| Wang P (2016) (34) | c-IA/B | Surgery: 106SBRT: 74Matched: each 35 | Lobectomy: 60.4%Sublobar: 39.6% | 60 Gy/3–8 fr: 90.5%54 Gy/3 fr: 9.5% | 69% (5y) | 44.6% (5y) | 67.8% (5y) | 47.4% (5y) | There were no differences in OS;SBRT is an alternative treatment option to surgery in elderly NSCLC patients who cannot tolerate lobectomy | |
| P=0.0007 | P=0.07 | |||||||||
Conclusions
Many studies using PSM analysis founded that SBRT can be an alternative treatment option to SLR in high-risk patients who cannot tolerate lobectomy because of medical comorbidities, but also as well as being an alternative to lobectomy. Randomized phase III trials comparing lobectomy or SLR with SBRT are warranted in the near future.
Acknowledgements
Funding: This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant No. 17K10478).
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.05.17). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Cancer Center Japan. Center for Cancer Control and Information Services. Available online: http://ganjoho.jp/reg_stat/statistics/stat/summary.html\
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN) Guidelines. Non-small cell lung cancer. Version 5. 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- The Japan Lung Cancer Society. Lung cancer practical guideline 2016. Available online: https://www.haigan.gr.jp/modules/guideline/index.php?content_id=3
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Timmerman R, Rebecca P, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Videtic GM, Hu C, Singh AK, et al. A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I non-small cell lung cancer. NRG Oncology RTOG 0915 (NCCTG N0927) Int J Radiat Oncol Biol Phys 2015;93:757-64. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small-cell lung cancer: Updated results of 257 patients in Japanease multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Yaes RJ, Patel P, Maruyama Y. On using the linear-quadratic model indaily clinical practice. Int J Radiat Oncol Biol Phys 1991;20:1353-62. [Crossref] [PubMed]
- Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011;81:e305-16. [Crossref] [PubMed]
- Kimura T, Nagata Y, Eba J, et al. A randomized Phase III trial of comparing two dose-fractionations stereotactic body radiotherapy (SBRT) for medically inoperable Stage IA non-small cell lung cancer or small lung lesions clinically diagnosed as primary lung cancer: Japan Clinical Oncology Group Study JCOG1408 (J-SBRT trial). Jpn J Clin Oncol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: Can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [Crossref] [PubMed]
- Timmerman R, Paulus R, Pass HI, et al. Stereotactic body radiation therapy (SBRT) to treat operable early stage lung cancer patients. ASCO 2013; Abstract.
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Eba J, Nakamura K, Mizusawa J, et al. Stereotactic body radiotherapy versus lobectomy for operable clinical stage IA lung adenocarcinoma: comparison of survival outcomes in two clinical trials with propensity score analysis (JCOG1313-A). Jpn J Clin Oncol 2016;46:748-53. [Crossref] [PubMed]
- Mokhles S, Verstegen N, Maat APWM, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: Results from propensity score analysis. Lung Cancer 2015;87:283-9. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015;99:1122-9. [Crossref] [PubMed]
- Rosen JE, Salazar MC, Wang Z, et al. Lobectomy versus stereotactic radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44-54.e9. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: Survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240-4. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, Welsh JW, Gomez DR, Swisher S, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060-70. [Crossref] [PubMed]
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non–small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014;147:1183-1191; discussion 1191-2. [Crossref] [PubMed]
- Matsuo Y, Chen F, Hamaji M, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: a propensity score matching analysis. Eur J Cancer 2014;50:2932-8. [Crossref] [PubMed]
- Zheng X, Schipper M, Kidwell K, et al. Survival Outcome After stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: A meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [Crossref] [PubMed]
- Port JL, Parashar B, Osakwe N, et al. A propensity-matched analysis of wedge resection and stereotactic body radiation therapy for early stage lung cancer. Ann Thorac Surg 2014;98:1152-9. [Crossref] [PubMed]
- Puri V, Crabtree TD, Bell JM, et al. Treatment outcomes in stage I lung cancer: A comparison of surgery and stereotactic body radiation therapy. J Thorac Oncol 2015;10:1776-84. [Crossref] [PubMed]
- Ma L. Xiang. Clinical outcomes of video-assisted thoracic surgery and stereotactic body radiation therapy for early-stage non-small cell lung cancer: A meta-analysis. Thorac Cancer 2016;7:442-51. [Crossref] [PubMed]
- Wang P, Zang D, Guo XD, et al. A propensity-matched analysis of surgery and stereotactic body radiotherapy for early stage non-small cell lung cancer in the elderly. Medicine 2016;95:e5723. [Crossref] [PubMed]
Cite this article as: Kimura T. Stereotactic body radiation therapy versus surgery for patients with stage I non-small cell lung cancer. AME Med J 2017;2:64.

