
Non selective beta-blocker in cirrhosis: not ‘whether’ but ‘who and how’
Non selective beta-blockers (NSBBs) have been used for decades in patients with liver cirrhosis to reduce portal pressure and the risk for portal hypertensive bleeding (1-3). International guidelines recommend NSBB as primary and secondary prophylaxis for variceal bleeding in cirrhosis (4). Patients with liver cirrhosis and portal hypertension have a distinct pathophysiological feature dominated by a hyperdynamic circulation (5). This arises primarily from a progressive splanchnic vasodilatation which causes an increased portal inflow and as a consequence a raised heart rate and cardiac index (5). NSBBs abrogate this mechanism by inducing a splanchnic vasoconstriction and reducing the inotropic and chronotropic competence of the heart thereby reducing the portal inflow and portal hypertension (6).
The protective effect of NSBBs correlates well with the reduction in heart rate during NSBB treatment. As this marker is non-invasive and simple to use it is used in clinical practice to titrate NSBB, either to a heart rate of 55–60 bpm or to the highest tolerated dose (4). Other non-invasive markers defining a treatment response have not been systematically evaluated and the hepatic venous pressure gradient (HVPG) is not frequently used, as invasive measurements are required.
In addition to this there are other NSBB related effect, which are independent of hemodynamic actions. NSBBs reduce the severity of bacterial translocation from the intestine into the systemic circulation. This is important as this mechanism has been shown to be up-regulated and substantially involved in inducing a systemic immune dysfunction in liver cirrhosis (6). It is therefore not surprising that NSBBs reduce the frequency of infections, such as the spontaneous bacterial peritonitis (7).
Once cirrhotic patients develop portal hypertension and varices, the disease is most progressive and patients have a high likelihood of decompensation with typical complications such as ascites, infection, bleeding and hepatic encephalopathy (8). As in these stages the hyperdynamic circulation is further enhanced one might expect that NSBBs exhibit an increasingly positive effect on portal hypertension.
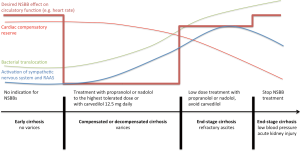
However, there is a growing body of evidence, that NSBB can also be harmful in end stages of liver cirrhosis. Sersté et al. (9) were the first to propose a reduced survival probability in patients with refractory ascites treated with NSBB. The same study group has shown a high risk for AKI with NSBB in a retrospective cohort of 139 patients with acute alcoholic hepatitis (10). In another small retrospective cohort of 94 patients with advanced stages of liver cirrhosis, NSBB was associated with a higher number of patients developing hepatorenal syndrome if they were in Child-Pugh stage C (11). Such data led to the development of the ‘window hypothesis’ (12), which proposed that there is only a short window in which NSBB are beneficial. In early stages of cirrhosis without clinically evident portal hypertension and hyperdynamic circulation, NSBB neither prevents the formation of varices nor impacts on patient survival. If the disease progresses the portal pressure increases and the sympathetic nervous system stimulates the cardiocirculatory system, however, with a preserved cardiac reserve. At this stage, NSBBs effectively abrogate the hyperdynamic circulation, reduce the portal pressure and improve patients’ survival. In end-stage cirrhosis complicated by refractory ascites or hepatorenal syndrome the cardiac response to stimuli such as bleeding, paracenteses or infections is diminished. The treatment window then closes as NSBB reduce patients’ survival and increase the risk for kidney injury (12).
The recently published study from Kim et al. (13) adds more evidence on the detrimental effect of NSBB on the development of complications, more precisely renal failure, in patients with end-stage cirrhosis. They performed a case-control study in patients awaiting liver transplantation due to cirrhosis between 1990 and 2010 in Rochester, USA. From a total cohort of 2,361 waitlist patients, 252 who developed acute kidney injury (AKI) were matched with 205 patients without AKI by variables that influence the occurrence of this complication (age, serum creatinine, MELD-Na). The objective was to assess the effect between NSBB exposure and AKI development. The frequency of NSBB use was 45.9% in the AKI group, which was insignificantly higher compared to 37.1% in the control group. In order to assess the effect of NSBB on AKI in different stages of cirrhosis they divided the cohort according to the presence of ascites and the administration of NSBB in four groups. After adjusting for demographic data and MELD in a multivariate model, NSBB increased the risk for AKI in patients with cirrhosis and ascites more the 3-fold (HR 3.31) whereas patients without ascites and NSBB had a risk reduction of about 5-fold (HZ 0.19). This study is well in line with the ‘window hypothesis’ that there is a certain clinical threshold, which is defined by Kim SG (13) as the occurrence of ascites, above which NSBB should be discontinued due to an unfavourable impact on the course of cirrhosis (12,13). This threshold has been proposed as point of no return after which haemodynamic compensatory mechanisms are irreversibly impaired and re-introduction of NSBB after the resolution of acute events such as hepatorenal syndrome should be avoided (14).
However, interrelationships between NSBB and end-stages of cirrhosis seem to be much more complex and study results on NSBB in end-stage cirrhosis are conflicting. There are several studies showing the opposite effect on patients’ survival with NSBB and end-stage cirrhosis. Despite the presence of (refractory) ascites (15-19) or severe liver dysfunction in acute-on-chronic liver failure (20) patients with NSBB had a significantly better survival. At this stage, it is unclear as to whether the prevention of portal hypertensive bleeding or the modulation of bacterial translocation or even both can elucidate this observation. However, there are two aspects, which have been inadequately considered in this debate so far. Njei et al. performed a meta-analysis showing that NSBB related mortality is dependent on the type of beta-blocker (21). Whereas conventional NSBBs such as propranolol and nadolol were not associated with an increased mortality in patients with all grades of ascites, carvedilol, which possesses an additional vasodilating intrinsic anti-alpha-1-adrenergic activity, decreased the survival probability with a number needed to treat of eight (21). Another interesting analysis has been published by Madsen et al. (22). They divided 81 patients with end-stage cirrhosis according to the NSBB dose in three groups (high-dose 160mg, low-dose 80mg, no NSBB). Whereas bleeding risks were similar among groups, patients with low-dose NSBB had a significantly better survival than patients with high dose or no NSBB.
All these results about the general survival benefit for NSBB in end-stage cirrhosis and dose and treatment type dependency suggest that the NSBB window is unlikely to be closed in end-stage cirrhosis and the question is therefore not about ‘whether’ to treat but rather ‘who and how’ to treat with NSBB. Most probably, we have to get away from a standardized treatment aiming at a maximum dose but to a low-dose and individualized treatment with conventional NSBB (Figure 1). (Bio)markers indicating the transition from benefit to detriment during NSBB treatment in cirrhosis would help to titrate NSBB in end-stage cirrhosis as the commonly used biomarker, heart rate is obviously inadequate. However, there are still patients with low blood pressure and/or hepatorenal syndrome, in which discontinuation of NSBBs is necessary (4).
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.06.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lebrec D, Poynard T, Hillon P, et al. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis. A controlled study. N Engl J Med 1981;305:1371-4. [Crossref] [PubMed]
- Pascal JP, Cales P, et al. Propranolol in the prevention of first upper gastrointestinal tract hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med 1987;317:856-61. [Crossref] [PubMed]
- Bhardwaj A, Kedarisetty CK, Vashishtha C, et al. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomized placebo-controlled trial. Gut 2016; [Epub ahead of print]. [Crossref] [PubMed]
- de Franchis R, Abraldes JG, Bajaj J, et al. Expanding consensus in portal hypertension: Report of the BAVENO VI Consensus Workshop: Stratifying risk and individualized care for portal hypertension. J Hepatol 2015;63:743-52. [Crossref] [PubMed]
- Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis 2014;18:281-91. [Crossref] [PubMed]
- Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol 2017;66:849-59. [Crossref] [PubMed]
- Senzolo M, Cholongitas E, Burra P, et al. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int 2009;29:1189-93. [Crossref] [PubMed]
- Ripoll C. Hepatic venous pressure gradient and outcomes in cirrhosis. J Clin Gastroenterol 2007;41:S330-5. [Crossref] [PubMed]
- Sersté T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010;52:1017-22. [Crossref] [PubMed]
- Sersté T, Njimi H, Degré D, et al. The use of beta-blockers is associated with the occurrence of acute kidney injury in severe hepatitis. Liver Int 2015;35:1974-82. [Crossref] [PubMed]
- Kalambokis GN, Baltayiannis G, Christou L, et al. Red signs and not severity of cirrhosis should determine non-selective beta-blocker treatment in Child-Pugh C cirrhosis with small varices: increased risk of hepatorenal syndrome and death beyond 6 month of propranolol use. Gut 2016;65:1228-30. [Crossref] [PubMed]
- Krag A, Wiest R, Albillos A, et al. The window hypothesis: haemodynamic and non-haemodynamic effects of beta-blockers improve survival of patients with cirrhosis during a window in the disease. Gut 2012;61:967-9. [Crossref] [PubMed]
- Kim SG, Larson JJ, Lee JS, et al. Beneficial and harmful effects of non-selective beta blockade on acute kidney injury in liver transplant candidates. Liver Transpl 2017;23:733-40. [Crossref] [PubMed]
- Ge PS, Runyon BA. When should the beta-blocker window in cirrhosis close? Gastroenterology 2014;146:1597-9. [Crossref] [PubMed]
- Leithead JA, Rajoriya N, Tehami N, et al. Non-selective beta-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut 2015;64:1111-9. [Crossref] [PubMed]
- Aday AW, Mayo MJ, Elliot A, et al. The beneficial effect of beta-blockers in patients with cirrhosis, portal hypertension and ascites. Am J Med Sci 2016;351:169-76. [Crossref] [PubMed]
- Bang UC, Benfield T, Hyldstrup L, et al. Effect of propranolol on survival in patients with decompensated cirrhosis: a nationwide study based Danish patient registers. Liver Int 2016;36:1304-12. [Crossref] [PubMed]
- Onali S, Kalafateli M, Majumdar A, et al. Non-selective beta-blockers are not associated with increased mortality in cirrhotic patients with ascites. Liver Int 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Sinha R, Lockman KA, Mallawaarachchi N, et al. Carvedilol use is associated with improved survival in patients with liver cirrhosis and ascites. J Hepatol 2017;67:40-6. [Crossref] [PubMed]
- Mookerjee RP, Pavesi M, Thomsen KL, et al. Treatment with non-selective beta-blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol 2016;64:574-82. [Crossref] [PubMed]
- Njei B, McCarty TR, Garcia-Tsao G. Beta-blockers in patients with cirrhosis and ascites: type of betablocker matters. Gut 2016;65:1393-4. [Crossref] [PubMed]
- Madsen BS, Nielsen KF, Fialla AD, et al. Keep the sick from harm in spontaneous bacterial peritonitis: dose of beta blockers matters. J Hepatol 2016;64:1455-6. [Crossref] [PubMed]
Cite this article as: Engelmann C, Jalan R. Non selective beta-blocker in cirrhosis: not ‘whether’ but ‘who and how’. AME Med J 2017;2:90.

