
Role of the reactive oxygen species induced DNA damage in human spermatozoa dysfunction
Introduction
DNA damage is a condition frequently observed in spermatozoa of subfertile male patients and associated with a variety of adverse pathological outcomes, such as impaired fertilization, disrupted preimplantation embryonic development, increased rates of miscarriage, and an enhanced risk of disease in progeny. DNA fragmentation is often caused by hyperproduction of reactive oxygen species (ROS) and considered to be one of the most important traits of oxidative stress. Particularly, in male germ cells it correlates with their dysfunction and evidences of impaired spermatogenesis. Oxidative stress is believed to impede spermatogenesis that leads to generation of cells with poorly remodeled chromatin (1-4). Male germ line is characterized by very little capacity to respond to the oxidative damage of DNA, because it only possesses the first enzyme in the base excision repair (BER) pathway, 8-oxoguanine glycosylase 1 (OGG1). This enzyme successfully creates an abasic site, but the spermatozoa cannot process the oxidative lesion further, because they lack downstream proteins, such as apurinic endonuclease 1 (APE1) and the X-ray repair cross-complementing protein 1 (XRCC1), which are needed to complete the repair process. Therefore, it is the responsibility of the oocyte to complete the reparation process before the newly formed zygote enters the S-phase of the first mitotic division (5,6). If DNA reparation systems of the oocyte work incorrectly, a mutation will be created and represented in every cell in the body. The defective spermatozoa tend to undergo apoptosis, the so called “programmed cell death”, which is associated with downward trend in metabolic activity, loss of motility, activation of caspases, increased generation of ROS by mitochondria, up-regulation of free radical oxidative processes and eventually fatal cell damage and death. It is believed that most of DNA damages in sperm cells have non-enzymatic etiology and caused by oxidative processes, because cytological organization of sperm cells prevents nuclease access to chromatin (6,7). Bearing in mind the important role of oxidative processes in DNA damage and spermatozoon malfunction one can expect an important role of antioxidants in treatment of these conditions.
In the last decade, frequency of human reproductive pathologies has been demonstrating steady upward trend. Approximately 1 in 20 of the male population of developed countries suffers infertility (5-7).
The International Committee Monitoring Assisted Reproductive Technologies (ICMART) reported total of 954,743 initiated cycles of in vitro fertilization (IVF) worldwide resulted in an estimated 237,809 babies born in 2004. This was a 2.3% increase in the number of reported cycles from 2003 (8).
It is suggested that between three and seven per cent of all couples worldwide have an unresolved problem of infertility. Far more couples, however, experience involuntary childlessness for at least 1 year: estimates range from 12% to 28% (9). Fertility problems affect one in seven couples in the UK. Women become less fertile as they get older. For women aged 35, about 94% who have regular unprotected sexual intercourse get pregnant after 3 years of trying. For women aged 38, however, only about 77%. The effect of age upon men’s fertility is less clear. In people going forward for IVF in the UK, roughly half of fertility problems with a diagnosed cause are due to problems with the man, and about half due to problems with the woman. However, about one in five cases of infertility has no clear diagnosed cause. In Britain, male factor infertility accounts for 25% of infertile couples (7). In Sweden, approximately 10% of couples wanting children are infertile. In approximately one third of these cases the man is the factor, in one third the woman is the factor, and in the remaining third the infertility is a product of factors on both parts (10). In Russia, only about 0.45% of annual fertilities are made by IVF (11). This is, however, seems likely to be due to poor availability of assisted reproductive technologies (ARTs) as nearly 50% of clinics are located in Moscow and St. Petersburg. This allows us to suggest that although, according to Russian medical specialists, the level of infertility in Russia barely exceeds 15%, this value may be, in fact, higher.
Non-enzymatic oxidative damage of DNA is known to provide a significant impact in male subfertility, and use of damaged spermatozoa for ART is associated with a number of clinical outcomes including miscarriage, disordered embryonic development and defects in offsprings. Several studies showed an increase in hospitalization of ART offsprings in comparison with naturally conceived children, whereas other investigations revealed abnormal retinal vascularization and eightfold increase in the incidence of undescended testicles in boys conceived by the intracytoplasmic sperm injection (ICSI) (12). Recently, a new technique has been proposed to increase the ART quality and overcome the obstacles mentioned above. This approach is based upon the microscopic selection of good quality spermatozoa under high magnification (over 6,000×) and is known as intracytoplasmic morphologically selected sperm injection (IMSI). However recent studies have showed that there is no evidence of effect on live birth or miscarriage and the evidence that IMSI improves clinical pregnancy is of very low quality and require further trials before recommending IMSI in clinical practice (13).
These defects are suggested to be, at least partly, due to poor repair of DNA lesions brought into the oocyte with a defective spermatozoon. These lesions may be introduced by a variety of factors including age, chemical agents, ionizing radiation, cryostorage, UV and oxidative stress. Moreover, recent studies revealed negative effect of Wi-Fi use on the sperm quality and spermatozoa capacitation (14). It was also shown that drugs used for chemical therapy of cancer also induce DNA fragmentation in spermatozoa (15).
However, it is known that the majority of such lesions are repaired by special DNA-reparation systems. Hence, it is the number and severity of DNA damage, on one hand, and the efficacy and fidelity of these systems on the other that will determine whether the DNA lesion will have any biological effect. Therefore, deeper understanding of the origins of such genomic damages is believed to be a challenging task in order to prevent their occurrence and develop effective approaches for their treatment and targeted therapy.
Factors causing DNA damage in germ line cells
Natural strand breaks
DNA fragmentation in male germ cells may occur during the process of normal spermatogenesis in order to relieve tension in DNA chain caused by its packaging into a very small head of the spermatozoon. Normally, these physiological strand breaks are repaired by a complex of process including H2Ax phosphorylation and the subsequent activation of nuclear poly(ADP-ribose) polymerase and topoisomerase (16). This is supposed to be a regulatory mechanism of spermatogenesis. Down-regulation of DNA reparation systems in spermatozoa may cause the inhibition of sperm cell generation and release of cells carrying damaged DNA.
Oxidative status and low antioxidant activity
Processes of free radical oxidation are known to cause damage of a variety of cellular structures including membranes, proteins and nucleic acids both in the nuclei and mitochondria. However, it is not still clear whether they account for the majority of DNA damages in human spermatozoa. Nevertheless, it goes without saying that specific features of male spermatozoa, such as active motility, require high mitochondrial activity and thus, the increased level of biological oxidation. Work of electron transportation chains in biological membranes, particularly mitochondria, is associated with production of ROS. Human spermatozoa are characterized by high capacity to generate ROS, such as superoxide anion and hydroxyl radical, and the level of ROS production demonstrates strong correlation between DNA strand breaks and the level of 8-hydroxy-29-deoxyguanosine (8OHdG), which is significantly elevated in the spermatozoa of subfertile patients (17,18). Model experiments, in which the motility of sperm cells was analyzed in the presence of ROS generated by xanthine oxidase, showed hydrogen peroxide to be the main cytotoxic agent, because it was catalase rather than superoxide dismutase that prevented the sperm motility loss (17,18). However, the pernicious influence of oxidative environment of the male germ line may be observed even under the conditions where motility is normal (5). Dose-dependent analysis of the influence of oxidative stress on sperm-oocyte fusion demonstrated a biphasic effect of ROS on the sperm function. It was shown that at low levels of oxidative stress, sperm-oocyte fusion rate was increased, possibly because of the positive role of ROS in driving the tyrosine phosphorylation reaction, which is associated with sperm capacitation, and sterol oxidation that facilitates cholesterol efflux from the sperm cell plasma membrane (5,19). However, at higher levels of oxidative stress the induction of lipid peroxidation in the plasma membrane is associated with a decline in the competence of sperm cells to fuse with the oocyte. This is apparently due to the direct oxidative damage of proteins involved in the fusion process (5,20,21).
However, when human spermatozoa were exposed to increasing levels of hydrogen peroxide it was not only the fertilizing potential and motility of the cells, but also DNA modifications that followed a biphasic pattern of change. At low levels of oxidative stress DNA damage was diminished, possibly because of the powerful effect of glutathione peroxidase (GPx) on chromatin cross-linking. Conversely, at high levels of oxidation, the sperm chromatin started to fragment (5,21). It is noteworthy, that DNA damage occurred at lower levels of oxidative stress as compared with the loss of fertilizing capacity of sperm cells. Thus, sperm that had been driven to a high readiness for fertilization by low levels of oxidative stress was also characterized by significant levels of DNA damages (5,21).
Sperm cells are characterized by high susceptibility to oxidative stress, because they possess an abundant level of targets for free radical oxidation, such as unsaturated fatty acids (about 50% of fatty acids in human spermatozoa is represented by docosahexaenoic acid, which contains six double bonds) and DNA in the nucleus and mitochondria. It was shown that sperm mitochondria respond to the presence of free unsaturated fatty acids with a dramatic increase in ROS generation, and the greater the level of unsaturation, the greater the level of stimulatory effect (5,21). This pro-oxidant effect is prevented by esterification of fatty acids. Therefore, it may be suggested that the central point in the induction of ROS generation by mitochondria is the amphiphilic properties of free fatty acids that define the orientation of the fatty acids in relation to the mitochondrial electron transport chain. In this context, it is noteworthy that defective human spermatozoa possess abnormally high amount of free polyunsaturated fatty acids, the levels of which positively correlates with generation of ROS in mitochondria (22). Moreover, products of lipid peroxidation, such as acrolein and some other small electrophilic aldehydes are also capable of triggering ROS generation by the sperm mitochondria (23). This capacity of lipid peroxidation products to stimulate mitochondrial ROS generation appears to be a function of their capacity to adduct onto proteins in the mitochondrial electron transport chain, such as succinate dehydrogenase (23). Abasalt et al. [2013] showed that mean concentration of MDA in seminal plasma and spermatozoa of infertile men was higher than in healthy normozoospermic subjects. The long-range PCR-analysis revealed multiple deletions in 64% of the case group patients and 44% of the control group. Moreover, MDA level in the spermatozoa of the deleted mtDNA samples group was significantly higher than in non-deleted mtDNA group (24). The authors imply that lipid peroxidation or other oxidative stress factors might be causative elements in mtDNA damage and effect on sperm motility and morphology. Aktan et al. [2013] studied the sperm DNA fragmentation and ROS formation efficacy with the terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) test and 2’,7’-dichlorodihydrofluorescein, respectively, in the semen samples of idiopathic infertile patients. Semen samples were also tested for the concentrations of plasma protein carbonyl groups, nitrotyrosine, MDA and the total level of SH-groups in order to study the antioxidant power of sperm (25). It was shown that the number of TUNEL-positive spermatozoa from idiopathic infertile men was higher than that from fertile men, and ROS formation was increased in infertile males as well. A positive correlation was detected between TUNEL-positive cells and ROS content (26). Seminal plasma MDA, the concentrations of plasma protein carbonyl groups, and nitrotyrosine levels were elevated in idiopathic infertile males. Seminal plasma MDA levels were shown to correlate positively with both concentration of plasma protein carbonyl groups and nitrotyrosine. Positive correlations were also observed between the level of DNA fragmentation and the concentrations of MDA, plasma protein carbonyl groups and nitrotyrosine in the seminal plasma, as well as between the levels of ROS and MDA in sperm (25).
Therefore, it is evident that oxidative stress in human spermatozoa is a self-propagating cycle that, once initiated, will inevitably lead to oxidative damage, malfunctions and eventually cell death.
On the other hand, these cells definitely lack intracellular antioxidant protection, because of low volume of cytoplasm, in which antioxidative enzymes, such as GPx, catalase and superoxide dismutase, fulfill their function. The main portion of spermatozoon cytoplasm is accumulated in the midpiece of the cell and has little access to DNA in the nucleus. Therefore, a considerable role in DNA protection in spermatozoa belongs to protamines, which form complexes with DNA (27). Moreover, DNA is so tightly packed in the sperm cell head that it can barely undergo reparation. Conversely, damages rather accumulate in the sperm cell DNA till fertilization and probably become subjects for correction only in the oocyte.
Another possible protection of the male germ line is the complex of extracellular antioxidants secreted in the male reproductive tract by other cells. For example, epididymis is known to produce extracellular superoxide dismutase and selenocysteine independent forms of GPx (28). It was shown that knock out of extracellular GPx encoding gene in mice led to the formation of age-related phenotype associated with the induction of oxidative DNA damage in the spermatozoa and the occurrence of birth defects in the offspring (29). Besides enzymatic antioxidants male reproductive tract also generates small antioxidant molecules, which work as free radical scavengers. The most important of these are vitamin C, uric acid, tryptophan, spermine and taurine. If the amount of these antioxidative substances is depleted for some reason, for example in case of heavy smoking, it leads to development of pro-oxidative potential in the male reproductive tract and consequently to DNA fragmentation in sperm cells. It was shown that 2-month treatment with vitamins E and C resulted in significant decrease in the level of DNA damage in human spermatozoa (30). Ozdemirler-Erata et al. [2013] demonstrated the defensive role of thioredoxin reductase (TR) against DNA fragmentation in spermatozoa of varicocele patients. It was shown that both activity and expression of TR were increased significantly in varicocele patients in comparison with fertile men (31). This suggests vitally important role of both enzymatic and non-enzymatic antioxidative systems in protection of male germ line against oxidative damages.
Excessive exposure to ROS
Besides low activity of enzymatic and non-enzymatic antioxidative systems, DNA fragmentation in male germ line may be also caused by excessive exposure to ROS. The latter may be generated either by spermatozoa themselves or by leukocytes, particularly neutrophils and macrophages, which are always present in sperm. It is well known that the efficacy of ROS generation by leukocytes is much higher than that of sperm cells. Therefore, the increased contamination of sperm with leukocyte cell subpopulations raises the risk of oxidative injury of spermatozoa. However, in subclinical amounts this leukocyte subpopulation has little effect on fertilization capacity of sperm (1). This may be due to the fact that the majority of seminal phagocytes originate from secondary sexual gland and have little contact with sperm cells prior to ejaculation. Moreover, as it was mentioned above, spermatozoa are effectively protected by antioxidative systems of seminal plasma that provide detoxication of leukocyte-derived ROS under natural conditions. However, this protection may fail in IVF cycles, when sperm undergo special preparation procedures affecting the properties of seminal plasma (1). It was shown that the disruptive effect of leukocytes in vitro can be reversed by supplementation of the medium with antioxidants, including reduced glutathione, N-acetylcysteine, hypotaurine and catalase (32). Therefore, the addition of antioxidants to the sperm preparation media used in ARTs would be a rational approach to circumvent this problem. On the other hand the pernicious oxygen metabolites may also be generated by spermatozoa themselves. It is believed that ROS generated by the male germ line originate either from mitochondria located in the midpiece of the cell or from the reaction catalyzed by the plasma membrane NADPH-oxidase (33). However, it was shown that spermatozoa injured by oxidation demonstrated enhanced production of ROS in mitochondria rather than up-regulation of NADPH-oxidase pathway. It appears that this activation of ROS generation by mitochondria of defective sperm cells is associated with initiation of apoptosis.
Role of mitochondrial ROS generation in apoptotic disruption of spermatozoa
In the absence of fertilization, most spermatozoa become senescent and default to an apoptotic state. Contrastingly to the somatic cells, the apoptosis of which is associated with extensive nuclear fragmentation, caused by the release of nucleases from mitochondria or their activation in the cytosol, the specific architecture of spermatozoa makes them different from all other cell types. One of the most important features is that in spermatozoa nucleus is physically separated from the mitochondria and most of the cytoplasm. Therefore, even when apoptosis is induced in suspensions of human spermatozoa, the DNA does not become cleaved by nucleases at least in the short term (34). The only products of apoptosis that can damage sperm DNA are the ROS generated by the mitochondria (5,34). Male germ line demonstrates a variety of apoptotic features, such as up-regulation of caspases 1, 3, 8 and 9, binding of annexin-V and generation of ROS in mitochondria (Figure 1). It was shown that treatment of human spermatozoa with a variety of apoptosis-inducing factors, including staurosporine, lipopolysaccharide, 3-deoxy-D-manno-octulosonic acid (Kdo) and genistein, failed to initiate the cell suicide program. However, the sperm cells, which undergo oxidative stress, demonstrated especially effective default to the intrinsic apoptotic pathway. Indeed, it was observed that exposure of human spermatozoa to H2O2 triggered an apoptotic cascade characterized by the activation of caspase 3 and the appearance of annexin-V binding activity. Moreover, pre-exposure of human spermatozoa to antioxidants, such as melatonin or catalase prevented this apoptotic response. Such an apoptotic cascade can also be precipitated by a variety of factors that induce oxidative stress in spermatozoa by triggering free radical generation by the mitochondria, including exposure to radiofrequency electromagnetic radiation, treatment with unsaturated fatty acids and dosing with the phosphatidylinositol-3-kinase inhibitor, wortmannin (21).
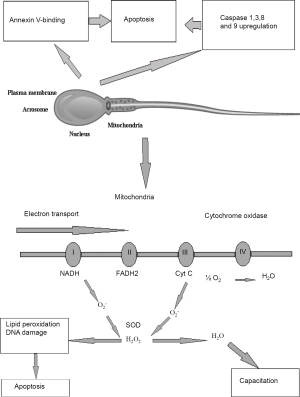
Since apoptosis cannot initiate immediate DNA fragmentation in a spermatozoon, like it does in somatic cells, and cannot immediately and completely deprive the sperm cells of fertilization capacity it is quite possible that sperm cells, which have just entered the apoptotic program, can still fertilize the oocyte in spite of DNA damage caused by oxidative attack (23). This suggestion was confirmed by the observation of association between DNA damage in the germ line and miscarriage and morbidity in the offspring (35). Taking into account the aforesaid features of physical structure of sperm cells, it is apparent that the only element of the apoptotic cascade in spermatozoa that can induce such damage is the ROS released from the mitochondria, and most of the DNA damage seen in human spermatozoa is oxidative in nature (5). It is, however, noteworthy that nucleases, if ever involved, might have the opportunity to impact on DNA fragmentation in spermatozoa either in the very beginning or the very end of a sperm cell lifecycle. Enzymatic cleavage of spermatid DNA takes place during the late spermatogenesis in order to relieve the torsional tension in the double helix and facilitate chromatin compartmentalization. These physiological nicks are believed to be further repaired prior to spermiation. However, if these reparation mechanisms fail, the nicked DNA may appear in mature sperm. Therefore, it may be suggested that both DNA fragmentation and poor chromatin compaction may be considered as independent factors causing errors in spermatogenesis. According to the “two-step” model, errors of the systems aimed at the reparation of nicks, which were introduced into the DNA during early spermatogenesis, result in poor chromatin protamination and, consequently, vulnerability of spermatozoa with respect to the damaging factors. In the second step, sperm cells undergo oxidative attack, which may be caused by either exogenous ROS produced, for example, by infiltrating leukocytes or by endogenous ROS produced by mitochondria, when entering the intrinsic apoptotic cascade (36).
However, in vivo sperm cells are expected to demonstrate high survival capacity, because it takes them nearly a week to pass through the epididymis lumen and one more week to persist in the female reproductive tract waiting for an oocyte to arrive. Contrastingly, it was shown that 12 h incubation of human spermatozoa at 37 °C caused a considerable motility loss associated with phosphatidylserine exteriorization, increase in caspase activity and DNA fragmentation (21). This means that the survival of spermatozoa in both male and female reproductive tracts should be sustained by certain prosurvival factors, which are absent in culture media. Moreover, signals produced by these survival factors should be transferred to the sperm cells and further into intracellular level via special receptors and signaling pathways. It was found out that the cell surface of spermatozoa carries around 20 different receptors. The most well studied among them is the prolactin receptor, which is present on the surface of human spermatozoon, particularly in the postacrosomal region of the sperm head, neck, midpiece, and principal piece of the sperm tail, in four different isoforms (one long form and three variants of the short form), one of which is unique to the sperm cells. It was shown that prolactin stimulates phosphatidylinositol 3-kinase (PI3K) activity, maintains protein kinase B (Akt/PKB) phosphorylation and has a powerful prosurvival effect on human spermatozoa (37). The authors demonstrated that prolactin inhibited sperm capacitation in a dose-dependent manner, suppressing SRC kinase activation and phosphotyrosine expression. The suppression of sperm capacitation was accompanied by a powerful prosurvival effect, supporting the prolonged motility of these cells and preventing the formation of spontaneous DNA strand breaks via mechanisms that involved the concomitant suppression of caspase activation. It was indicated that the prosurvival effect of prolactin on human spermatozoa involved the stimulation of Akt phosphorylation, whereas inhibitors of PI3K and Akt negated this effect. Similar result was obtained by direct stimulation of sperm capacitation with cAMP analogues. The authors concluded that prolactin works as a prosurvival factor for human spermatozoa that prevents these cells from defaulting to an intrinsic apoptotic pathway associated with cell senescence (37). It is most likely that prolactin is not the only prosurvival factor of human spermatozoa. Nevertheless, study of the mechanisms of its prosurvival effects revealed fundamental role of PI3K/Akt-signaling pathway in this process. Incubation of spermatozoa in vitro in the absence of prosurvival factors induces them to default to the apoptotic way associated with increased ROS generation by mitochondria, accumulation of 8OHdG adducts and, finally, DNA fragmentation. Therefore, further studies of prosurvival factors of spermatozoa are considered to be promising with respect to development of optimized media for cultivation and storage of sperm cells used in ART.
Basically, there are two main reasons for spermatozoa to undergo apoptosis in vivo. First, it is necessary to remove dead, moribund and damaged sperm cells, which failed to fertilize the egg from the female reproductive tract. The other reason is to avoid oocyte fertilization with a spermatozoon, which has already defaulted to apoptosis. In any case woman’s organism meets millions of sperm cells, which have to be eliminated from the reproductive tract. This attracts massive infiltration of leukocytes into the cervix and uterus in order to destroy moribund spermatozoa by phagocytosis. However, this phagocytic activity must be silenced so as to avoid inflammation. Therefore, apoptosis seems to be the most suitable way to eliminate damaged spermatozoa, because cells defaulting to the apoptotic pathway are characterized by expression of apoptotic markers, such as phosphatidylserine, on their surface. It is believed that these markers provide signals to phagocytes that the target cell should be engulfed in a non-phlogistic manner (38). The expression of apoptotic markers may be considered as clear indicator that the spermatozoon is the subject for disposal. Hence, it is drastic to select spermatozoa for ICSI on the basis that they do not express markers of apoptosis, such as annexin-V binding or phosphatidylserine. This seems to be a rational approach towards reducing the risk that DNA-damaged spermatozoa are used in ART.
Role of PI3K/Akt-signaling pathway in regulation of male germ line lifespan
One of the most important protective mechanisms against premature apoptosis in human spermatozoa is the PI3K/Akt-signaling pathway that transfers prosurvival signals from the cellular surface to the nucleus. This pathway, like many others, is activated by Ras protein and involves two major kinases PI3K and Akt/PKB (Figure 2).
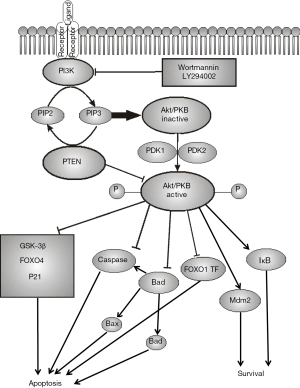
PI3K is activated through binding to the SH2-domain of the activated receptor and further with Ras GTPase. Activated PI3K phosphorylates 3’-OH groups of phosphatidylinositol 4,5-bisphosphate (PIP2) to form phosphatidylinositol 3,4,5-trisphosphate (PIP3). The phosphorylated inositol residues exposed on the inner surface of the plasma membrane can bind downstream proteins to form complexes required for further signal transduction. The key role in this process belongs to PIP3, which can specifically bind the pleckstrin homology (PH) domain containing proteins, such as phospholipase C (PLC), Akt/PKB (serine-threonine kinase) and Bruton tyrosine kinase (BTK). It is believed that the key protein in the PH-domain containing protein subfamily is Akt/PKB, which binds PIP3-residues on the cytoplasmic side of the plasma membrane. After that, Akt/PKB undergoes double phosphorylation by the kinases PDK1 and PDK2 that leads to its activation and further phosphorylation of a series of downstream signaling factors and eventually regulatory effects on the transcription factors and target genes. Among the most important biological effects of Akt/PKB is the increase of cell survivability and suppression of apoptosis, stimulatory effect on cell proliferation and growth, and possibly activation of cell motility (Table 1). The effects of PI3K/Akt/PKB are finely controlled by phosphatases, such as PTEN, which removes the 3’-phosphate group from PIP3 to get back PIP2 and thus, counters the effects of Akt/PKB activation.
Table 1
| Biological effect | Akt/PKB substrate | Result |
|---|---|---|
| Inhibition of apoptosis | Bad (pro-apoptotic) | Inhibition |
| Caspase-9 (pro-apoptotic) | Inhibition | |
| IκB (antiapoptotic) | Activation | |
| FOXO1 TF(pro-apoptotic) | Inhibition | |
| Mdm2 (antiapoptotic) | Activation | |
| Proliferation | GSK-3β (antiproliferative) | Inhibition |
| FOXO4 (antiproliferative) | Inhibition | |
| p21Clp1(antiproliferative) | Inhibition | |
| Growth | Tsc2 (anti-growth) | Inhibition |
PKB, protein kinase B.
As it was already mentioned above, the specific features of structural organization of spermatozoa prevents them from being committed to the classical apoptotic cascade typical for somatic cells. Moreover, germ line, contrastingly to the somatic line, lacks the cell-cycle checkpoints and transcriptional activity, and thus can default to the truncated intrinsic apoptosis, which is characterized by motility loss, oxidative DNA damage and dephosphorylation of PI3K/Akt (34).
Western blot analysis with anti-PI3K/Akt polyclonal antibodies clearly demonstrated the presence of these kinases in human spermatozoa (34). Immunohistochemical analysis showed that in human spermatozoa PI3K was located mostly in the principal piece of the sperm tail, the neck and the acrosome, but not in the midpiece, where the mitochondria and residual cytoplasm are located. Akt was shown to be present in human spermatozoa in two isoforms, Akt1 and Akt2, the former being located throughout the sperm tail, including the principal piece and the midpiece, and the latter being strongly present in the midpiece and, to a lesser degree, in the proximal part of the flagellar principal piece. However, contrastingly to Akt1, Akt2 was also found in the sperm head, particularly in the vicinity of the acrosome (34).
Importantly, the phosphorylated and stabilized form of PTEN was shown to be located in the equatorial segment of the sperm head that is distant from the PI3K in the principal piece of the tail. This physical separation of PI3K and PTEN enables spermatozoa to generate abundant amounts of PIP3 and avoid the premature commitment to apoptosis (34). The authors showed that initiation of apoptosis in human spermatozoa by treatment with PI3K inhibitors, wortmannin and LY294002, resulted in the formation of dephosphorylated pro-apoptotic Bad, dose- and time-dependent loss in the sperm motility, increase in the mitochondrial ROS generation, phosphatidylserine externalization and activation of caspases (34). Dephosphorylation of Bad is known to allow Bax and Bak to fore pro-apoptotic pores in the outer mitochondrial membranes (39). As soon as the phosphorylation status of PI3K/Akt is compromised, sperm cells default to the intrinsic apoptotic pathway associated with Bad dephosphorylation and increase in mitochondrial permeability. Hence, dephosphorylation of Bad was followed by the increased mitochondrial ROS generation, cytochrome c release, caspase activation, phosphatidylserine externalization, cytoplasmic vacuolization and other traits of intrinsic apoptosis initiation (34,40). Neither wortmannin nor LY294002 induced translocation of nucleases or any other effectors of apoptotic cascade, such as cytochrome c or DIABLO, from the midpiece to the nucleus. However, wortmannin was shown to increase oxidative damage of DNA in dose-dependent manner. Finally, activation of the intrinsic apoptotic cascade with wortmannin resulted in vacuolization and membrane protrusions in the sperm cell midpiece (34).
Therefore, the lifespan of a sperm cell depends drastically on the mechanisms, which regulate the PI3K/Akt signaling. In normal conditions, balance in the activities of PI3K/Akt and PTEN is supposed, on one hand, to enable spermatozoa to survive long enough to meet and fuse with the oocyte, and to provide fast removal of aged and damaged sperm cell from the woman’s reproductive tract on the other. Although the PI3K/Akt signaling cascade is considered to be the key regulator of the sperm cell survivability, it is most likely that it is not the only mechanism that is aimed at the protecting of sperm from undergoing premature apoptosis, and search of other mechanisms opens a wide field for further investigations.
Summary
Problems of male fertility caused by spermatozoa malfunction are believed to be one of the important factors of involuntary childlessness in contemporary World. One of the key factors, inducing decrease in fertilization capacity is DNA damage. Most of such damages occur non-enzymatically, as the cytological architecture of spermatozoa impedes enzymatic attack on DNA, and to a significant degree due to the ROS produced in cells. In fact, DNA strands may be broken even during normal spermatogenesis as DNA needs to be packed into a very small head of spermatozoon. However, normally, such breaks are repaired by special mechanisms of DNA reparation that include H2Ax phosphorylation and the subsequent activation of nuclear poly(ADP-ribose) polymerase and topoisomerase (16). The specific functional activities of spermatozoa, such as, for example, active motility, require high mitochondrial activity and, thus, increased level of ROS generation. This provides the basis for the idea that free radical oxidation processes may play a key role in non-enzymatic damage of DNA. The outcome of free radical oxidation on DNA damage in spermatozoa depends on the balance of the excessive exposure to ROS and efficacy antioxidative systems. It was found out that sperm cells, which were exposed to low level of oxidative stress, were characterized by increased fusion capacity with respect to oocytes. This effect is, apparently, due to the positive role of ROS in tyrosine phosphorylation, sterol oxidation and subsequent efflux of cholesterol from the plasma membrane that is associated with spermatozoon capacitation. Nevertheless, higher levels of oxidative activity led to the lipid peroxidation and, eventually, resulted in decline in the spermatozoon competence to fuse with the oocyte. Sperm cells, which underwent a low level of oxidative stress, were characterized by insignificant damages of DNA, possibly, due to the GPx effect on chromatin cross-linking, whereas higher levels of oxidative stress led to chromatin fragmentation. Notably, DNA damages were observed at the levels of oxidative stress lower than it is required to deprive a sperm cell of fertilizing capacity. Therefore, the sperm cells, the fertilizing activity of which was induced by mild oxidation, were characterized by noticeable level of DNA damage (5). Defective spermatozoa are characterized by abundant level of free polyunsaturated fatty acids, which were shown to induce generation of ROS in mitochondria (22). It is believed that free radical oxidation processes may affect sperm motility and fertilization capacity via modifications of mtDNA (24). It was also demonstrated that spermatozoa and seminal plasms of idiopathic infertile patients contained higher levels of MDA, plasma protein carbonyl groups and nitrotyrosine as compared with fertile men. Hence, it seems likely that oxidative stress plays an important role in development of sperm infertility and malfunction. Moreover, the cytological architecture of sperm cells itself makes it problematic to provide the sufficient intracellular level of antioxidants, because small volume of cytoplasm. Apparently, antioxidant machinery of oocytes corrects damages in male line DNA after fertilization. Another possible protection of the male germ line is the complex of antioxidative enzymes, such as superoxide dismutase and selenocysteine independent GPx, and low molecular weight scavengers of free radicals, such as vitamin C, uric acid, tryptophan, spermine and taurine secreted in the male reproductive tract (1,28).
One of the most important protective mechanisms against premature apoptosis in human spermatozoa is the PI3K/Akt signaling pathway. It was found out that PI3K/Akt and PTEN antagonists are located in different compartments of a sperm cell that makes them physically separated prom each other. This allows the spermatozoon to produce an abundance of PIP3 and avoid premature apoptosis (34). Treatment with PI3K inhibitors, such as wortmannin and LY294002, resulted in induction of intrinsic apoptosis, associated with sever oxidative damage of mitochondrial and nuclear DNA.
Therefore, it is crucial to develop target approaches to stimulate antioxidative capacity of male germ line and up-regulate antiapoptotic signaling mechanisms in order to prevent and treat male infertility.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.01.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl 2011;13:36-42. [Crossref] [PubMed]
- Talebi AR, Ghasemzadeh J, Khalili MA, et al. Sperm chromatin quality and DNA integrity in partial versus total globozoospermia. Andrologia 2018;50: [PubMed]
- Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev 2017;84:1039-52. [Crossref] [PubMed]
- Aitken RJ. DNA damage in human spermatozoa; important contributor to mutagenesis in the offspring. Transl Androl Urol 2017;6:S761-S764. [Crossref] [PubMed]
- Aitken RJ, Smith TB, Jobling MS, et al. Oxidative stress and male reproductive health. Asian J Androl 2014;16:31-8. [Crossref] [PubMed]
- Agarwal A, Majzoub A. Role of Antioxidants in Assisted Reproductive Techniques. World J Mens Health 2017;35:77-93. [Crossref] [PubMed]
- Almeida S, Rato L, Sousa M, et al. Fertility and Sperm Quality in the Aging Male. Curr Pharm Des 2017;23:4429-37. [Crossref] [PubMed]
- Sullivan EA, Zegers-Hochschild F, Mansour R, et al. International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: assisted reproductive technology 2004. Hum Reprod 2013;28:1375-90. [Crossref] [PubMed]
- Hammarberg K, Collins V, Holden C, et al. Men's knowledge, attitudes and behaviours relating to fertility. Hum Reprod Update 2017;23:458-80. [Crossref] [PubMed]
- Wosnitzer M, Goldstein M, Hardy MP. Review of Azoospermia. Spermatogenesis 2014;4:e28218 [Crossref] [PubMed]
- Safronova LD, Kudriavtsev IV, Kudriavtsev PI. Sterility of males determined by functional features of the mouse spermatozoa bearing t-complex. Ontogenez 2002;33:165-9. [PubMed]
- Finnström O, Källén B, Lindam A, et al. Maternal and child outcome after in vitro fertilization--a review of 25 years of population-based data from Sweden. Acta Obstet Gynecol Scand 2011;90:494-500. [Crossref] [PubMed]
- Teixeira DM, Barbosa MA, Ferriani RA, et al. Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database Syst Rev 2013;CD010167 [PubMed]
- Akdag MZ, Dasdag S, Canturk F, et al. Does prolonged radiofrequency radiation emitted from Wi-Fi devices induce DNA damage in various tissues of rats? J Chem Neuroanat 2016;75:116-22. [Crossref] [PubMed]
- Baysal M, Ilgin S, Kilic G, et al. Reproductive toxicity after levetiracetam administration in male rats: Evidence for role of hormonal status and oxidative stress. PLoS One 2017;12:e0175990 [Crossref] [PubMed]
- Meyer-Ficca ML, Lonchar J, Credidio C, et al. Disruption of poly(ADP-ribose) homeostasis affects spermiogenesis and sperm chromatin integrity in mice. Biol Reprod 2009;81:46-55. [Crossref] [PubMed]
- Gosalvez J, Tvrda E, Agarwal A. Free radical and superoxide reactivity detection in semen quality assessment: past, present, and future. J Assist Reprod Genet 2017;34:697-707. [Crossref] [PubMed]
- Anbari F, Halvaei I, Nabi A, et al. The quality of sperm preparation medium affects the motility, viability, and DNA integrity of human spermatozoa. J Hum Reprod Sci 2016;9:254-8. [Crossref] [PubMed]
- Brouwers JF, Boerke A, Silva PF, et al. Mass spectrometric detection of cholesterol oxidation in bovine sperm. Biol Reprod 2011;85:128-36. [Crossref] [PubMed]
- Rao M, Zhao XL, Yang J, et al. Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian J Androl 2015;17:668-75. [Crossref] [PubMed]
- Aitken RJ, Gibb Z, Baker MA, et al. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev 2016;28:1-10. [Crossref] [PubMed]
- Koppers AJ, Garg ML, Aitken RJ. Stimulation of mitochondrial reactive oxygen species production by unesterified, unsaturated fatty acids in defective human spermatozoa. Free Radic Biol Med 2010;48:112-9. [Crossref] [PubMed]
- Aitken RJ, Whiting S, De Iuliis GN, et al. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem 2012;287:33048-60. [Crossref] [PubMed]
- Abasalt HC, Gholamali JS, Maryam GC. Lipid peroxidation and large-scale deletions of mitochondrial DNA in asthenoteratozoospermic patients. Indian J Biochem Biophys 2013;50:492-9. [PubMed]
- Aktan G, Doğru-Abbasoğlu S, Küçükgergin C, et al. Mystery of idiopathic male infertility: is oxidative stress an actual risk? Fertil Steril 2013;99:1211-5. [Crossref] [PubMed]
- Ribeiro S, Sharma R, Gupta S, et al. Inter- and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology 2017;5:477-85. [Crossref] [PubMed]
- Bennetts LE, Aitken RJ. A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol Reprod Dev 2005;71:77-87. [Crossref] [PubMed]
- Agarwal A, Roychoudhury S, Bjugstad KB, et al. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol 2016;8:302-18. [Crossref] [PubMed]
- Chabory E, Damon C, Lenoir A, et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J Clin Invest 2009;119:2074-85. [PubMed]
- Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 2011;26:1628-40. [Crossref] [PubMed]
- Özdemirler Erata G, Küçükgergin C, Aktan G, et al. Is thioredoxin reductase involved in the defense against DNA fragmentation in varicocele? Asian J Androl 2013;15:518-22. [Crossref] [PubMed]
- Duhig K, Chappell LC, Shennan AH. Oxidative stress in pregnancy and reproduction. Obstet Med 2016;9:113-6. [Crossref] [PubMed]
- Chauvigné F, Boj M, Finn RN, et al. Mitochondrial aquaporin-8-mediated hydrogen peroxide transport is essential for teleost spermatozoon motility. Sci Rep 2015;5:7789. [Crossref] [PubMed]
- Koppers AJ, Mitchell LA, Wang P, et al. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J 2011;436:687-98. [Crossref] [PubMed]
- Aitken RJ, Bronson R, Smith TB, et al. The source and significance of DNA damage in human spermatozoa; a commentary on diagnostic strategies and straw man fallacies. Mol Hum Reprod 2013;19:475-85. [Crossref] [PubMed]
- Castillo J, Simon L, de Mateo S, et al. Protamine/DNA ratios and DNA damage in native and density gradient centrifuged sperm from infertile patients. J Androl 2011;32:324-32. [Crossref] [PubMed]
- Pujianto DA, Curry BJ, Aitken RJ. Prolactin exerts a prosurvival effect on human spermatozoa via mechanisms that involve the stimulation of Akt phosphorylation and suppression of caspase activation and capacitation. Endocrinology 2010;151:1269-79. [Crossref] [PubMed]
- Kurosaka K, Takahashi M, Watanabe N, et al. Silent cleanup of very early apoptotic cells by macrophages. J Immunol 2003;171:4672-9. [Crossref] [PubMed]
- Polzien L, Baljuls A, Rennefahrt UE, et al. Identification of novel in vivo phosphorylation sites of the human proapoptotic protein BAD: pore-forming activity of BAD is regulated by phosphorylation. J Biol Chem 2009;284:28004-20. [Crossref] [PubMed]
- Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol 2008;83:288-95. [Crossref] [PubMed]
Cite this article as: Bibov MY, Kuzmin AV, Alexandrova AA, Chistyakov VA, Dobaeva NM, Kundupyan OL. Role of the reactive oxygen species induced DNA damage in human spermatozoa dysfunction. AME Med J 2018;3:19.

