
Proton pump inhibitor non-responsive gastroesophageal reflux disease: unraveling an enigma?
Gastroesophageal reflux disease (GERD) affects 10–20% of adults in Western countries (1,2) and 13% of Americans use medications for GERD at least twice weekly (3). In the USA alone, it is estimated that the annual direct and indirect costs incurred due to GERD is approximately $10 billion (4).
Proton pump inhibitors (PPIs) have been the gold standard treatment of GERD for over two decades. In multiple studies, PPIs have been shown to be superior over placebo and histamine-2 receptor antagonists for the control of symptoms, esophageal mucosal healing, and improvement in quality of life (2). However, despite this success, it has been estimated that approximately 10–40% of patients with GERD do not respond adequately to PPIs in standard doses (1,4). Recently, more attention has been focused on these PPI non-responsive (PPINR) patients and how best to treat them. PPI non-responsive GERD is typically defined as patients who have objective evidence of reflux (either esophagitis or abnormal pH study) despite at least 8-week trial of PPI therapy (2,4,5). Due to sparse evidence of the effectiveness of adjunctive therapies, there is no standard diagnostic or treatment approach to patients with PPINR GERD, which has created a sea of confusion and patients often getting referred to surgical anti-reflux procedures.
In a recent issue of the Diseases of the Esophagus, Hillman et al. present a narrative review of medical therapy options for PPINR GERD (6). They divide their review into two main segments: (I) the efficacy of CYP independent vs. CYP dependent PPIs in participants with different metabolizing potential; and (II) the efficacy of medications in addition to PPIs in the treatment of GERD. Hillman et al. should be praised for their meticulous work on this subject.
The authors reviewed 13 studies that compared the efficacy of PPIs based on subject’s CYP metabolization genotype (6). Subjects that were homozygous for the rapid CYP2C19 metabolizer gene were known as the homozygous extensive metabolizer (homoEM) group, while the ones with slower CYP2C19 metabolizer gene were characterized as the poor metabolizer (PM) group, and patients with one of each gene comprised the heterozygous extensive metabolizer (heteroEM) group. In the five studies that compared the effects of the more CYP-dependent PPIs (lansoprazole, pantoprazole, and omeprazole) on subjects based on their genotype, the poor metabolizers generally had better outcomes, with both better symptomatic relief and higher rates of remission of erosive esophagitis, as compared to the heteroEM and homoEM groups. The heteroEM group also fared better compared to the homoEM group. The differences between the groups were no longer significant in the one study where the PPI dosing was increased to twice a day (6).
Six other studies compared the efficacy of the more CYP-independent PPIs (rabeprazole and esomeprazole) among the different genotypes and found that the symptom relief and erosive esophagitis healing rates were not significantly different (6). Lastly, when comparing response between CYP2C19 dependent and independent PPIs, evidence was conflicting with one study showing that resolution of symptoms at 4 weeks did not differ across genotypes, while another showing decrease recurrence rate in patients treated with rabeprazole compared to CYP-dependent PPIs (omeprazole or lansoprazole) (6). Studies have shown that for symptom relief, esomeprazole (CYP-independent PPI) is more rapidly effective compared to CYP dependent PPIs such as omeprazole (7,8), lansoprazole (9,10), and pantoprazole (11). Two meta-analyses have shown that esomeprazole was superior to omeprazole and other PPIs for healing esophagitis at four and 8 weeks (12,13). However, these studies primarily included patients that were treatment naïve and did not evaluate patients with PPINR GERD.
When reviewing the benefits of adjunctive medications in the treatment of GERD, Hillman et al. found mixed results. The authors specifically reviewed 27 studies that analyzed multiple other medical therapies in conjunction with PPI use. Histamine-2 receptor antagonist (H2RA) use in addition to PPI did decrease nocturnal acid breakthrough and nighttime as well as overall symptoms compared to PPI use alone, but many subjects experienced tachyphylaxis and stopped taking H2RAs, limiting the clinical applications (6).
Studies analyzing adjunctive promotility agents (mosapride, prucalopride, revexepride, and domperidone) were also reviewed (6). Among all GERD patients, mosapride showed no differences compared to PPI monotherapy, however, in the PPI non-responsive group specifically, symptoms improved with mosapride plus PPI (6). Prucalopride was only studied in a case report of four subjects with constipation and GERD and did show improvements in symptoms. Revexpride + PPI, on the other hand, did not show any improvements in symptoms compared to PPI monotherapy among PPINR participants (6). Domperidone + PPI was helpful in providing symptom relief while patients were on the medications, but differences between the groups disappeared post-treatment (6). In a previous randomized, placebo-controlled trial, metoclopramide and domperidone did not improve duration of esophageal acid exposure or clearance despite both agents significantly increasing the lower esophageal sphincter pressure (14).
Finally, mucosal protective agents (rebapamide, irsogladine, and mirgeal) in addition to PPIs showed some benefit compared to PPI alone (6). Rebapamide + PPI reduced symptom recurrence rate of EE that had been treated with PPIs (6). Irsogladine + PPI did not show improvements in symptoms across all groups but did show improvement over PPI alone in a subgroup of NERD patients without microscopic changes on biopsy. Mirgeal also demonstrated improved symptom control with PPI in patients with NERD (6).
This study has several limitations. First, this systematic review included studies that enrolled participants irrespective of how the diagnosis of GERD was made (including self-reported symptoms or patient reported questionnaires). As the authors acknowledged, this was due to paucity of studies with clearly defined objective evidence of PPI refractory GERD, but regardless, it increases risk of potentially including patients that might have been misclassified as GERD. Second, studies comparing the efficacy of CYP independent and dependent PPIs draws mainly from studies conducted in Japan and Taiwan. Only one of the 13 studies reviewed was conducted in Europe and there were no studies from the United States. Given higher prevalence of rapid metabolizers (homoEM) among Caucasians (59.7–69.9%) compared to Asian populations, one would expect greater benefit with CYP independent PPIs in the Western population (15). Third, studies evaluating efficacy of CYP polymorphism on PPI effectiveness were primarily studied in patients with severe reflux (erosive esophagitis). Evidence behind using this approach among patients with non-erosive reflux disease (NERD) is unclear. Finally, evidence of other pharmaceutical options is lacking as noted by the authors due to lack of uniform studies (including medication dosing, follow-up period, and the method by which the diagnosis of refractory GERD was made). We propose the following algorithm (Figure 1) in patients with PPINR GERD based on current literature with most important delineation being testing to prove objective evidence of reflux. The current American College of Gastroenterology (ACG) guidelines recommend that patients with low pre-test probability (atypical presentation without concomitant heartburn or regurgitation) of reflux be tested OFF medication with pH or multi-channel intraluminal impedance-pH (MII-pH), while those with high pre-test probability (typical symptoms of heartburn/regurgitation or partial response to PPI) of reflux be tested with MII-pH ON therapy (2). If ambulatory reflux testing is negative, then further work-up should be focused towards identifying alterative etiology given nearly 35% of patients with symptoms of GERD in one study had an alternative diagnosis on further evaluation (5.5% had eosinophilic esophagitis, 8.3% had achalasia or another dysmotility disease, 16% had functional heartburn, and 5.8% had gastroparesis) (5).
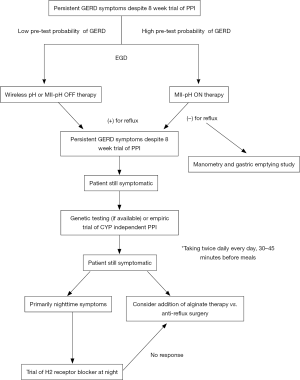
Hillman et al. (6) have made an important contribution in highlighting the current evidence behind treatment for patients with PPI non-responsive GERD. More importantly, this study reminds us that our current literature critically lacks high-quality outcomes based studies in this difficult to treat group.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Admir Kurtcehajic (Medical Center Plava Poliklinika, Tuzla, Bosnia and Herzegovina).
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.12.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther 2010;32:720-37. [Crossref] [PubMed]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-28; quiz 329. [Crossref] [PubMed]
- Sontag SJ. The medical management of reflux esophagitis. Role of antacids and acid inhibition. Gastroenterol Clin North Am 1990;19:683-712. [PubMed]
- Bashashati M, Hejazi RA, Andrews CN, et al. Gastroesophageal reflux symptoms not responding to proton pump inhibitor: GERD, NERD, NARD, esophageal hypersensitivity or dyspepsia? Can J Gastroenterol Hepatol 2014;28:335-41. [Crossref] [PubMed]
- Galindo G, Vassalle J, Marcus SN, et al. Multimodality evaluation of patients with gastroesophageal reflux disease symptoms who have failed empiric proton pump inhibitor therapy. Dis Esophagus 2013;26:443-50. [Crossref] [PubMed]
- Hillman L, Yadlapati R, Thuluvath AJ, et al. A review of medical therapy for proton pump inhibitor nonresponsive gastroesophageal reflux disease. Dis Esophagus 2017;30:1-15. [PubMed]
- Kahrilas PJ, Falk GW, Johnson DA, et al. Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. The Esomeprazole Study Investigators. Aliment Pharmacol Ther 2000;14:1249-58. [Crossref] [PubMed]
- Richter JE, Kahrilas PJ, Johanson J, et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol 2001;96:656-65. [Crossref] [PubMed]
- Castell DO, Kahrilas PJ, Richter JE, et al. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol 2002;97:575-83. [Crossref] [PubMed]
- Fennerty MB, Johanson JF, Hwang C, et al. Efficacy of esomeprazole 40 mg vs. lansoprazole 30 mg for healing moderate to severe erosive oesophagitis. Aliment Pharmacol Ther 2005;21:455-63. [Crossref] [PubMed]
- Labenz J, Armstrong D, Lauritsen K, et al. A randomized comparative study of esomeprazole 40 mg versus pantoprazole 40 mg for healing erosive oesophagitis: the EXPO study. Aliment Pharmacol Ther 2005;21:739-46. [Crossref] [PubMed]
- Edwards SJ, Lind T, Lundell L. Systematic review of proton pump inhibitors for the acute treatment of reflux oesophagitis. Aliment Pharmacol Ther 2001;15:1729-36. [Crossref] [PubMed]
- Gralnek IM, Dulai GS, Fennerty MB, et al. Esomeprazole versus other proton pump inhibitors in erosive esophagitis: a meta-analysis of randomized clinical trials. Clin Gastroenterol Hepatol 2006;4:1452-8. [Crossref] [PubMed]
- Grande L, Lacima G, Ros E, et al. Lack of effect of metoclopramide and domperidone on esophageal peristalsis and esophageal acid clearance in reflux esophagitis. A randomized, double-blind study. Dig Dis Sci 1992;37:583-8. [Crossref] [PubMed]
- McColl KE, Kennerley P. Proton pump inhibitors--differences emerge in hepatic metabolism. Dig Liver Dis 2002;34:461-7. [Crossref] [PubMed]
Cite this article as: Evers L, Patel D. Proton pump inhibitor non-responsive gastroesophageal reflux disease: unraveling an enigma? AME Med J 2018;3:21.

