Bronchial thermoplasty: an update for the interventional pulmonologist
Introduction
Asthma is one of the most common pulmonary diseases affecting 7–10% of the population (1). Its global prevalence, morbidity, mortality and economic burden has increased rapidly over the past few decades (2). Despite improvements in asthma care with different worldwide initiatives and development of new therapies, the number of hospital admissions related to asthma has increased, reflecting poor disease management and limited access to healthcare, as well as an increase in the severity of asthma (3).
Uncontrolled severe asthma could be life-threatening and affects the patient’s quality of life by limiting their physical, emotional, social and professional development (3). Its impact on society is significant, both due to increasing time off from work or school and rising the economic cost associated with its care (4). Patients with severe asthma represent only 10% of asthmatics (4,5). However, they are responsible for 50% of all directs and indirect costs (3). Therefore, patients from this specific group require individualized clinical assessment and medical management (4).
In the last two decades, bronchoscopic interventions such as bronchial thermoplasty (BT) for patients with severe asthma have been extensively studied in several multi-center trials (6,7). Although there is increasing data supporting its short- and long-term safety and effectiveness, BT has not been fully embraced by the medical community, due to (I) the challenges associated with categorizing patients with severe asthma in the daily clinical practice; (II) the difficulty of identifying the correct patient phenotype who will benefit the most from this procedure; and (III) the lack of understanding of the complex mechanism of action that BT exerts beyond the medium size airways.
The goal of this review is fourfold: (I) to provide the pulmonologist and bronchoscopist the rationale and mechanism of action of BT; (II) to help to identify the correct patient phenotype who will benefit from BT; (III) to review the technical aspects of the procedure; and (IV) to summarize the clinical trials that supports its effectiveness and safety.
Defining severe asthma in daily clinical practice
Accurate assessment of asthma severity is fundamental to predict risk of exacerbation, prevent complications and guide treatment. Multiple definitions for severe asthma have been proposed (NHLBI-NAEPP-3, ERS-ATS, GINA, BLAISS) (4,8-11). The clinician may encounter some challenges while trying to apply some of these criteria during his/her daily encounters with patients (e.g., performing outpatient pulmonary function tests in persistent symptomatic patients (NHLBI- NAEPP, ERS-ATS), collecting data from prior year related to medication and hospital admissions, or relying on questionnaire data (ERS-ATS) that might be in some cases subjective. Recently, a panel of asthma experts created a simple, unified definition of severe asthma that can be easily implemented in clinical practice. This group of experts defined severe asthma as: “asthma that, despite patient adherence, requires high doses of inhaled corticosteroids (ICS) plus long acting B-agents (LABA) and/or additional controller medication or requires oral corticosteroids (OCS) to prevent it from becoming uncontrolled or that remains uncontrolled despite therapy” (4).
It is key to remember that before classifying the severity of asthma, the clinician should have addressed medication adherence, side effects and access to treatment, inhaler technique, avoidance of allergenic agents, and other co-morbidities that might mimic or complicate asthma treatment such as smoking, esophageal reflux, allergic rhinitis, vocal cord pathology, or heart failure (4).
What is BT?
BT is a minimally invasive bronchoscopic intervention for carefully selected patients with severe asthma that delivers controlled thermal energy to the airway wall during a series of bronchoscopic procedures. More than 8,000 patients have undergone BT treatment globally. Clinical trials have demonstrated a reduction in future healthcare utilization and improved patient quality of life by decreasing asthma symptoms.
Rationale for BT
Mechanism of action
The mechanism of action of BT is complex and not completely understood. BT affects the airway smooth muscle (ASM), the extracellular matrix, airway innervation, the process of mast cell infiltration in the ASM layer, and the activation and recruitment of inflammatory cells (12) (Figure 1).
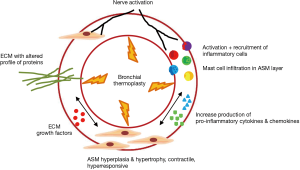
Airway remodeling is one of the hallmarks of asthma and is defined as alterations in the nature, content and organization of the cellular and molecular elements of the airway wall (12,13). This phenomenon contributes to: (I) the development of an irreversible component of airway obstruction and persistence of airway responsiveness; (II) loss of protective smooth muscle stretch relaxation and lung elastic recoil; and (III) reduction of airway distensibility and bronchodilator response (14). The initial concept of BT was to reduce ASM mass in the treatment area by inducing thermal ablative effects on the ASM leading to decreased bronchial constriction and improving patient symptoms (14,15). This hypothesis originated from BT study on canine model, airway responsiveness and ASM changes were noted up to 3 years post-treatment. ASM reduction is inversely correlated to airway responsiveness (i.e., more ASM reduction correlates to less airway hyper-responsiveness). Histology analysis showed normal epithelia layer at 12 weeks post-BT and absence of any scaring at 3 years. Reduction of ASM and airway responsiveness is sustained at 3 years (16). Encouragingly, similar findings were confirmed in a human cohort (17) (Figure 2). However, this is not the only effect BT has on ASM area. At least in animal experiments, ASM exposure to extreme temperatures (>55 °C) leads to complete inhibition of the contractile ASM function, prior to necrosis or apoptosis (18). Thus, suggesting that besides the absolute reduction of ASM mass, there may be a functional interference of ASM via BT treatment. Of note, BT thermal energy heat the airway tissue in a controlled manner to 65 °C.
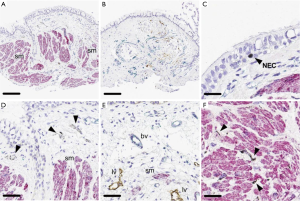
Pretolani et al. conducted a prospective study (n=10) in which patients with severe asthma undergoing BT would have bronchoscopy with trans-bronchial biopsies 15 days before and 3 months after the initial and third BT session respectively. This study found a decrease in ASM area of 65% before and after BT, and unexpectedly, a decrease of ASM area in the untreated right middle lobe was also observed. The latter finding suggested the possibility of heat diffusion generated during the BT procedure to adjacent lung parenchyma and more distal areas (19). Furthermore, the authors observed the development of ground glass opacities in both untreated and treated BT areas. This finding was subsequently confirmed in a separate study (20). Interestingly, Debray et al. reported the development of parenchymal consolidations, pleural effusions and fissure thickening in CT chest obtained 24 hours after BT treatment. All these changes resolved on subsequent imaging (20).
ASM cells are an important source of extracellular matrix inflammatory cytokines, chemokines and grow factors (12). By decreasing ASM mass, BT decreases the source of these mediators of inflammation and growth. Thus, resulting in the decrease of mesenchymal cells that produce type I collagen beneath the basal membrane and reduced recruitment of various inflammatory cell types (12,21,22). Cholinergic and non-adrenergic, non-cholinergic (NANC) innervations systems play a key role in the bronchomotor/bronchial tone regulation and inflammation of the airways (12). Recently, Pretolani et al. demonstrated that BT also decreased the amount of submucosal nerves and ASM-associated nerves, even in non-treated areas (right middle lobe) (17).
All these data suggest that the effects of BT are beyond the visible area of treatment and might extend into the small airways and alveolar area. Likewise, it could explain partially the increase of asthma exacerbations immediately post BT treatment (14). More studies are needed to confirm these potential mechanisms of action, and clarify if the acute parenchymal and pleural changes observed on imaging following BT might have any clinical consequences.
Summary of clinical evidence
A total of five large clinical trials involving BT were done in the last decade. Four of the five have extended follow-up data. A brief summary of the largest randomized controlled clinical trials of BT is found in Table 1. Here we will briefly describe the clinically most important trials.
Table 1
| Study | Design and objectives | Population | Key findings |
|---|---|---|---|
| Long-term outcomes of BT comparing two prospective studies (PAS2 vs. AIR2 trial) (7) | Prospective, open label, observational, multicenter study to assess long-term effectiveness of BT in patients with more severe disease than AIR2 trial | 190 patients from PAS2 trial (actively enrolling) vs. 190 BT patients from AIR2 trial. PAS2 trial patients were older, more obese and on higher doses of ICS compared to AIR2 patients | At year 3 after bronchial thermoplasty, the percentage of PAS2 subjects with severe exacerbations, ED visits and hospitalizations significantly decreased by 45%, 55% and 40%, respectively, resembling the AIR2 results |
| AIR2 extension study (23) | Prospective, multicenter study to assess the effectiveness and safety of BT in severe asthma patients 5 years after therapy | 162 patients from AIR2 trial followed over a period of 5 years | Effectiveness maintained long-term, demonstrated by sustained reduction in % of patients having severe exacerbation and ED visits. CT chest from baseline compared to 5 years after BT showed no structural abnormality secondary to BT. FEV1 values remained stable between years 1 and 5 after BT |
| AIR2 trial (6) | Prospective, multicenter, randomized, double blind, sham-controlled trial to assess effectiveness and safety in patients with severe asthma | 190 BT severe asthma patients and 98 sham control group. All patients were symptomatic despite high-dose ICS and LABA | Patients undergoing BT experienced an improvement from baseline in the integrated AQLQ score compared with sham. BT patients had higher risk of being hospitalized in the treatment period (up to 6 weeks after BT). After this and up to 1 year, the BT group experienced fewer severe exacerbations, ED visits, and days missed from work/school compared with the sham group (PPS, 95.5, 99.9, and 99.3%, respectively) |
BT, bronchial thermoplasty; PAS2, post-FDA approval clinical trial evaluating bronchial thermoplasty in severe persistent asthma; AIR2, asthma intervention research 2 trial; ED, emergency department; FEV1, forced expiratory volume during first second; ICS, inhaled corticosteroids; LABA, long acting beta-agonist.
AIR2 trial
Asthma Intervention Research Trial 2 (AIR2) was a multi-center, randomized, double blind, sham-controlled study that aimed to evaluate the effectiveness and safety of BT (6). The trial included 288 patients with severe asthma who were still symptomatic despite maximal medical therapy: ICS (doses greater than 1,000 ug/day of beclomethasone or equivalent) and LABA (doses at least of 100 ug/day of salmeterol or equivalent). The primary effectiveness endpoint was to assess the improvement of quality of life measured by the Asthma Quality of Life Questionnaire (AQLQ) score, as well as its safety. This study demonstrated clinically meaningful differences with the BT group being superior to the sham groups based in the following findings:
- AQLQ: 79% of patients in the BT group and 64% of patients in the sham group achieved a clinically meaningful improvement in the asthma quality of life (AQL), as measured in the AQLQ score change from baseline ≥0.5.
- Reduction of severe exacerbations: There was a 32% of reduction in severe exacerbations requiring systemic corticosteroids per patient/year in the BT group (0.48 versus 0.70, BT and sham group respectively).
- Emergency room (ER) visits and time lost from work: When the BT and sham group were compared; there was an 84% reduction in ERs visits for respiratory symptoms and 66% reduction in time lost from work/school/other daily activities due to asthma, favoring the BT group.
While the reduction of the AQLQ score reflects BT effectiveness in patients with severe asthma, the other endpoints (severe exacerbations, ER visits, and time lost for work) could be interpreted as both effectiveness and safety measurements (the latter, based on the fact that an intervention leads to a measurable decreased in the rate of adverse events). Although BT is a safe procedure, it should be noted that the AIR2 trial found an increase in peri-procedure respiratory adverse events in the BT group (from the first BT treatment until 6 weeks after the third treatment), including: asthma exacerbations (ten patients), low FEV1 (one patient), upper and lower respiratory tract infections, atelectasis (two patients), and hemoptysis (one patient, treated with bronchial embolization). There were no events of pneumothorax, intubations, mechanical ventilation, airway stenosis or bronchial narrowing, cardiac arrhythmias or deaths as a result of BT or sham bronchoscopy Importantly, 16 patients (8.4%) of the BT group required 19 hospitalizations for respiratory symptoms, more than half of them (10/19) occurring on the day of the procedure. All these findings suggest careful post procedure assessment and follow up of patients treated with BT is required (6).
AIR2 trial extension study
In order to assess the durability of effectiveness and safety of BT out to 5 years, Wechsler et al. followed the BT group on completion of the first year after the AIR2 trial (23). This study confirmed the long-term benefits to at least 5 years of BT as evidenced on the following end-points:
- Severe exacerbations (decrease of both event rates and patients with severe exacerbations, 48% and 44%, respectively), when compared with the 12 months sham group prior to BT treatment.
- ER visits for respiratory symptoms (88% average decrease) over 5 years in the ratio ER lists for respiratory symptoms compared with 1 year prior to BT treatment.
Regarding the long-term safety of BT, the trial showed that the FEV1 following BT remained unchanged in the BT group. Most importantly, there was no increase of the percentage of rate of respiratory adverse events or hospitalizations for respiratory symptoms, nor evidence of structural changes in the airways (as evaluated by chest tomography) at 5 years that were of clinically significance (23).
Long term outcomes of BT in the real world—comparing the PAS2 and the AIR2 trial
The PAS2 registry has enrolled 284 patients with a follow-up up to 5 years post BT. In order to evaluate the long-term durability and real-world effectiveness of BT, Chupp et al. compared the first 190 PAS2 patients to the 190 AIR2 BT-treated subjects at 3 years of follow-up (7). Notably, the PAS2 cohort was “sicker” than the AIR2 patients: older, higher BMI, higher rates of severe exacerbations and hospitalizations, and higher ICS dose/chronic use of OCS. At year 3 after BT, the PAS2 patients had significant reduction in severe exacerbations, ER visits, and hospitalizations compared to baseline (45%, 55% and 40% respectively, all P<0.005), resembling the AIR2 results. Thus, the PAS2 data suggest that the treatment effect of BT is both consistent and durable despite enrolling patients who had worse asthma control (7).
Current guidelines recommendations regarding BT
Based on current literature, the British Thoracic Society Guidelines in 2016 and the Global Initiative for Asthma (GINA) in 2017 recommended that BT may be considered for the treatment of adult patients who have poorly controlled asthma despite optimal therapy (Grade A and Evidence B respectively) (7,8). These recommendations are in line with prior guidelines published in 2014 by ERS/ATS Task Force on Severe Asthma that recommended BT in the same group but restricted to clinical studies or in the context of an independent Institutional Review Board-approved systematic registry (11). The American College of Chest Physicians in a position statement related in 2014, recognized the role of BT in carefully selected patients with severe asthma and that it should not considered experimental nor withheld from patients pending additional clinical trials (24).
Patient perspective
As mentioned before, uncontrolled severe asthma significantly impacts the quality of life of those patients effected, limiting their participation at work or school, and as functional individuals in society (3). Challenges of treating patients with uncontrolled severe asthma include poor medication adherence (25), the limited efficacy of the majority of current asthma drugs despite highest doses of complex regimens, and the multiple side effects associated with higher doses and long-term use of asthma drugs and OCS (9,26-28). Complications related to chronic use of OCS deserves further discussion. It is well-known that the development of complications with OCS use has a direct relationship with the chronicity of OCS use (>6 months), as well as OCS dose (odds ratio for low <5 mg/day, medium 5–10 mg/day, and high >10 mg/day of OCS exposure were 2.03, 2.85, and 3.64, respectively, versus OCS non-users) (29). In addition to the development of OCS related complications, it is important to highlight the economic impact on patient care cost when OCS complications occurs, adding an estimated annual healthcare costs for OCS users of $2,712 to $8,560 above those of OCS non-users (30).
Economic perspective
Although the proportion of patients with uncontrolled severe asthma represent only 10% of all asthmatics patients, this group is responsible for the significant portion of healthcare economic burden (4,5). This cost is related to the higher rates of asthma exacerbations per patient, increased morbidity and disproportionate use of healthcare resources (ER visits, hospitalizations, unscheduled physician visits, etc.). in this population. In the United States, a patient with severe asthma carries $2,325/year in direct costs to the healthcare system, almost twice the cost of a patient with non-severe asthma (31). In multiple recent studies, BT has been found to be cost effective (32-35).
Practical aspects of BT: patient selection
Asthma is a heterogeneous and complex chronic disease. Some patients will respond effectively to treatment, while others with refractory and severe disease might require further work-up and alternative treatments. A prior study in asthmatic patients identified two clusters of severe asthma that were clinically characterized by severe airflow obstruction with bronchodilator response, but both differed in age of onset, gender, atopy and use of OCS (36). More recently, the concept of the inflammatory sub-phenotypes of asthma has emerged by evaluating the sputum inflammatory cell profile in asthmatic patients (37). This study showed that patients with severe asthma and sudden-onset fatal asthma had neutrophil predominance and did not respond well to steroids, in contrast to their counterparts who had eosinophilic predominance (>2% of induced sputum eosinophils) and were responsive to steroids (37).
Induced sputum has not been extensively used to guide asthma treatment despite guidelines recommendations (9,11). This is largely due to lack of lab availability, duration of the analysis, and failure to obtain samples due to bronchoconstriction in severe asthmatic patients (38,39). These issues led to the search of another surrogate test to identify eosinophilic asthma. Recent evidence showed that blood eosinophils had higher accuracy in the identification of sputum eosinophilia (ROC AUC 89%, P<0.001), performed better than fractional exhaled nitric oxide (FeNO) and serum periostin (ROC AUC 78% and 55%, respectively) (38).
Asthma phenotyping would facilitate individualized treatment and rational management, and it should be carried out early when the asthmatic patient becomes difficult to treat and before the regular use of OCS, in order to prevent clinical deterioration and further complications related to systemic steroids (4,40). The work-up should include:
- Serum immunoglobulin E (IgE) (total and specific) in order to: (I) identify allergic asthma; (II) avoid allergenic triggers; and (III) identify patients who might benefit from anti-IgE therapy (omalizumab).
- Blood eosinophil count (absolute) with the goal to identify both inflammatory eosinophilic asthma and patients who might benefit of anti-IL5 therapy (mepolizumab, reslizumab or benralizumab).
- Induced sputum cellularity to assess airway inflammation, since it might reveal other inflammatory cell profile such as neutrophilic, mixed granulocytic and pauci-granulocytic asthma which are not able to be evaluated with blood samples (41).
Regarding the testing of FeNO in severe asthma patients, the ERS/ATS do not recommend its routine use based on the available evidence and its cost/benefit ratio (11).
Only after thorough clinical evaluation, work-up, and ensure maximal treatment compliance, the patient should be considered a candidate for BT. BT should be offered as a first option to patients with non-allergic, non-eosinophilic (non-T2) severe asthma, who have persistent symptoms and bronchodilator reversibility after failure of triple therapy with high-dose of ICS plus LABA and LAMA, but before regular OCS use. It could be offered as well to those patients with allergic or eosinophilic asthma who have failed targeted biologic therapy (4).
Practical aspects of BT: technique
BT is completed in three separate treatments, spaced 3 weeks apart, sequentially targeting right middle/lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is no longer avoided since the theoretically concern for right middle lobe syndrome has not been seen in over 8,000+ patients treated. Each treatment involves activation of Alair® catheter in contiguous, non-overlapping fashion, moving from distal to proximal airway (3 to 10 mm in diameter), moving systematically from airway to airway. Length of the procedure depends on the number of activations performed, but usually last less than 1 hour (Figure 3). Of note, the Alair catheter will fit a 2.0-mm working channel; thus, ideally should be used with a diagnostic (5.2 mm OD) or hybrid bronchoscope (4.2 mm OD). Larger bronchoscopes (>5.2 mm OD) are not recommended due to the limited access to smaller airways. The number of activations is related to the number of visible airways; hence, the smaller bronchoscope often correlates to higher number of activations. In a recent report, the number of activations predicted responder vs. non-responder to BT. Thus, one may consider that the use of the hybrid bronchoscope is more desirable due to smaller dimensions and therefore will achieve more visibility of the distal airways and resulting in increased number of activations (42) (Figure 4).
Indications
BT is FDA approved for severe persistent asthma in patients 18 years and older whose asthma is not well controlled with ICS and LABA. The signs of poorly controlled asthma are severe asthma attacks, or altering lifestyle to avoid triggers, or absence from work, school, or daily activities due to severe asthma symptoms despite maximal medical therapy (43).
Patient selection is a key consideration. As mentioned previously, the ideal patient is one with non-allergic, non-eosinophilic (non-T2) severe asthma, who has persistent symptoms and bronchodilator reversibility despite triple therapy with high-dose of ICS plus LABA and LAMA. Additionally, patient with refractory allergic or eosinophilic asthma who are already on targeted biologic therapy and are not responding to the therapy. These patients may stand to benefit the most from BT.
Contraindications
BT is not for use in patients under the age of 18, or have an active implantable electronic device (i.e., pacemaker, internal defibrillator, etc.), or known sensitivity to medications used in bronchoscopy (i.e., lidocaine, atropine, or benzodiazepines). Previously treated airways with BT should not be retreated as to avoid possible airway scaring and stricture. Patients should be stable and suitable to undergo bronchoscopy. The most common side effect of BT is an expected transient increase in the frequency and worsening of respiratory-related symptoms.
Anesthesia considerations
BT can be performed under moderate sedation or general anesthesia. Choice of anesthesia should be individualized to the patient and operate in accordance to each institution protocols. For example, it would be unwise to perform BT under moderate sedation on a patient with high opioid or benzodiazepine dependence. A recent report has found that moderate sedation with propofol and remifentanil TCI is safe and resulted in high satisfaction rates in both patients and bronchoscopists (44). In a separate report, a tertiary healthcare center examined their practice of anesthesia used in BT. They reported that while initial sets of BT treatments were done under moderate to deep sedation, the procedure was often interrupted with episodes of hypoventilation and/or airway obstruction that required interventions, such as jaw thrusts or oral/nasal airways. When the authors transitioned to general anesthesia with the use of a laryngeal mask airway (LMA®) or endotracheal (ET) tube, the procedural conditions were much improved compared to moderate and deep sedation (45). Hence, while BT may be done under moderate sedation or general anesthesia, one needs to take into considerations patient’s prior medication use, body habitus, projected procedural length (directly correlated to the number of activations), whether positive pressure is required to distend open the distal airways, and bronchoscopist’s comfort level. It is advisable that the focus of the bronchoscopist should be on planning and performing the BT procedure methodically (as described below), his/her attention should not be strained by administering anesthesia to the patient.
Procedural details
As stated previously, the entire BT treatment involves 3 separate bronchoscopy sessions spaced 3 weeks apart. The divided treatment reduces procedural length, anesthesia time, and risk of severe asthma exacerbation. Per standard protocol, first bronchoscopy targets the right middle/lower lobe, the left lower lobe is treated in the second bronchoscopy. Bilateral upper lobes are treated in the final bronchoscopy session.
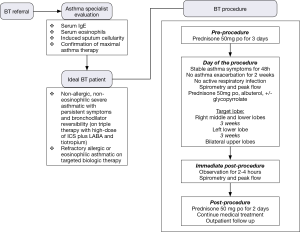
Before each bronchoscopy sessions, patients undergo the following preparation to further reduce the risk of an asthma exacerbation and ensure procedural success. To minimize post-procedure airway inflammation, patients are placed on prophylactic prednisone or equivalent at a dosage of 50 mg/d for the 3 days before the procedure, the day of procedure, and the day after the procedure. Albuterol and an antisialogogue agent such as glycopyrrolate should be administered a minimum of 30 minutes before the procedure. Pretreatment with albuterol nebulizer 2.5 to 5.0 mg and glycopyrrolate 0.2 to 0.4 mg IV/IM will help to optimize the reactive airway for BT and reduce secretions. Careful monitoring of the potential tachyarrhythmias or adverse central nervous system effects should be performed.
On the day of the bronchoscopy, patient should be evaluated for compliance with 3 days of prednisone therapy, ensure SpO2 is greater than 90% on room air, document stable asthma symptoms in last 48 hours, assess postbronchodilator FEV1 is more than 85% of pretreatment value, and document that there is no active respiratory infection nor were there asthma exacerbations within the past 2 weeks. These are to ensure baseline clinical status prior to BT as the treatment carries a short-term risk of asthma exacerbation.
Once appropriate level of anesthesia has been achieved, the bronchoscope is introduced to first inspect patient’s airway anatomy. Special attention should be paid to any previously treated airways to evaluate for possible mucus impaction and the degree of healing. After the initial inspection is completed, the bronchoscopist will focus on how to systematically approach the airways intended to be treated. Methodically moving from distal to proximal, working from airway to airway (from left to right, superior to inferior, in a clockwise fashion) across the region of lung being treated to ensure that all airways are treated once. The bronchoscope is placed at the most distal region of the first airway to be treated, ensuring clear bronchoscopic view, then the Alair® catheter is advanced via the working channel until the distal end is in bronchoscopic view. The electrode array with 4 electrode wires are expanded to contact the airway wall, caution must be used to avoid over-expanding and distorting the electrode array. Once visual confirmation is achieved, catheter is activated via pressing the footswitch. The energy is delivered automatically for approximately 10 seconds to preset treatment parameters reaching 65 °C. After each activation, electrode array is partially collapsed and repositioned proximally about 5 mm, in non-overlapping fashion, adjacent to the prior activation site. This process is repeated for the entire length of the airway. Anatomic landmarks are useful as reference points to help navigate systematically across different airways. It is also advisable to use a map to keep track of which airways have been treated and how many activations were done per airway (46). Of note, BT experts will often treat the airways beyond visually accessible by pushing the catheter beyond the limit of visualization until resistance is noted. Catheter is then sequentially activated every 5 mm by measuring the distance withdrawn at the proximal insertion site of the catheter into the working channel. Using this approach, one can achieve 100–150 activations per treatment.
Post-procedure care
Immediate post-procedure care is depended on individual institution guidelines. However, it is prudent to monitor patients carefully and discharged only when stable vital signs with adequate lung function (via spirometry), mental status, and able to take liquids. Post-procedure assessment should include evaluation of gag reflex, vital signs, breath sounds, and spirometry to assess FEV1. Typically, a 2 to 4 hours recovery or monitoring period following each procedure is needed. An objective measure to use for discharge after BT is when post-bronchodilator FEV1 is within 80% of the pre-procedure value and patient is feeling well clinically. Finally, it is important to stress the use of prednisone after the procedure and the adherence to prior asthma regimen.
Follow up
As with any bronchoscopic procedure on reactive airways, transient worsening of respiratory-related symptoms in the period immediately after BT is expected. Symptoms such as breathlessness, wheeze, cough, chest discomfort, night awakenings, and productive cough are often reported. These symptoms typically present in the first week after bronchoscopy and usually resolve with standard medical care within one week. Thus, close follow-up in the first week is encouraged. Patient should be contacted 24 hours, 48 hours, and 7 days post-procedure to assess their respiratory status. Of note, increased mucus production can occur in post-treatment period resulting in mucus plugging. Thus, chest physiotherapy and/or therapeutic bronchoscopy may be required for treatment. In considering the potential long-term role for BT in the treatment of asthma, the short-term risk of increased respiratory-related symptoms should be weighed against the potential for improvement in asthma control (43).
Future directions
As reviewed earlier, inflammation and smooth muscle dysfunction are essential components of severe asthma. Both processes contribute to airway luminal narrowing causing airflow limitation, ventilation heterogeneity and clinical symptoms. The treatment decisions, directed at inflammatory (i.e., anti-Th2 monoclonal antibodies) and non-inflammatory or smooth muscle-mediated (i.e., BT) components of severe asthma, are ideally guided by appropriately characterizing the asthma phenotype. In a recent study, Svenningsen et al. examined patients with controlled and uncontrolled sputum-eosinophil (marker of airway inflammation) and the ventilation response to salbutamol as measured by inhaled-hyperpolarized-gas MRI. The authors noted that ventilation heterogeneity as measured by MRI persists in post-salbutamol patients with uncontrolled eosinophilic-bronchitis suggesting that the airways are remodeled leading to these defects and would benefit from targeted BT (47). When this technique was applied to healthy and asthma patients (pre- and post-BT), Thomen et al. showed that regional quantification of lung ventilation is feasible and may be a useful technique for image-guided treatment of asthma with BT (48).
Furthermore, clinic trials with BT demonstrate peri-procedural asthma exacerbation usually in the same day as BT treatment. This occurs independent of bronchoscopy (as the increased rate of exacerbation was not observed in the sham-control arm) (6). Since only one region of the lung is treated at a time, this suggest that regional trigger of asthma exacerbation. Perhaps asthma exacerbation starts in the most vulnerable regions of the lung and then propagates to incorporate other portions of the lung. Therefore, using various imaging modalities, such as 129Xe and 3He MRI, to measure ventilation defect percentage (VDP) to define the specific defective ventilated area of the lung in severe asthma can lead to targeted regional treatment (49). Furthermore, recent developments in polarized optical coherent tomography (OCT) allowed for the first time to measure smooth muscle mass in vivo. ASM mass can now be quantified in patients with severe asthma in a real-time fashion (50). Thus, it is not inconceivable that in the near future, a patient with severe asthma can be stratified via phenotype, prior to undergo BT, a hyperpolarized gas MRI can be done to identify the specific lung region with defective ventilation, BT can then be performed with OCT guidance to identify the specific airway with most ASM mass within the area of ventilation defect. Of note, this targeted BT therapy may lead to reduction of the number of treatment needed (i.e., perhaps not all lobes of the lung need to be treated with BT to achieve maximal benefit).
Finally, airway hyper-responsiveness and ASM hypertrophy may ultimately fall in a spectrum of the disease evolution. It is analogous to diastolic heart failure where the myocardium undergoes significant remodeling and hypertrophy under chronic stress of hypertension. In the airway, the ASM undergoes hypertrophy and may become less able to relax; thus, resulting in loss of bronchial dilator response. BT can reduce the ASM mass and lead to improved symptom control. Additionally, it is now recognized that the ASM contributes significantly in synthetic function involving modulation of extracellular components, the local immune environment, and interactions with other airway cell types such as epithelium, fibroblasts, and nerves. Thus, by decreasing ASM component in the asthmatic airway, one will observe the combined effects of both decreased structural and synthetic functions (12,14,17). These areas are of significant clinical interest and will need to be explored further with future studies.
Key points
- A thorough evaluation of patient medication compliance, inhaler technique and associated co-morbidities should be performed before classification of asthma and consideration of BT.
- BT should be offered as a treatment option to non-allergic, non-eosinophilic (non-T2) severe asthma patients, who have persistent symptoms and bronchodilator reversibility after failure of triple therapy (high-dose ICS/LABA and LAMA), but before regular OCS or use of targeted biologic drug.
- In selected patients with severe uncontrolled asthma, BT has been proven to be safe and effective in the long term in multiple, large well-designed multi-center studies.
- Optimize procedural conditions with appropriate pre-treatment, anesthesia use, and bronchoscope will result in higher satisfaction from both the patient and the bronchoscopist.
- Bronchoscope size and positioning is correlated to the number of activations achievable, the higher number of activations is correlated with a better BT response.
Conclusions
BT is a minimally invasive intervention reserved for patients with uncontrolled severe asthma. Its effectiveness and safety have ample clinical evidence in several well-designed multi center trials. More studies are needed to elucidate the phenotype of severe asthma patient who will benefit from it and fully understand its complex mechanism of action.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Amit Mahajan, Sandeep J. Khandhar and Erik E. Folch) for the series “Management of Complex Airway and Pleural Diseases” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.07.10). The series “Management of Complex Airway and Pleural Diseases” was commissioned by the editorial office without any funding or sponsorship. M Castro currently receives University Grant money from NIH, American Lung Association. He currently receives pharmaceutical grant monies from: AstraZeneca, Chiesi, Novartis, Boehringer-Ingelheim, Sanofi-Aventis. He has received speaker/advisor fees from AstraZeneca, Aviragen, Boston Scientific, Genentech, Mallinckrodt, Nuvaira, 4D Pharma, Teva and Vectura. Royalties have been received from Elsevier. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rowe BH, Voaklander DC, Wang D, et al. Asthma presentations by adults to emergency departments in Alberta, Canada: a large population-based study. Chest 2009;135:57-65. [Crossref] [PubMed]
- CDC. Asthma: most recent asthma data. 2017. Available online: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed 12 March, 2018.
- Braman SS. The global burden of asthma. Chest 2006;130:4S-12S. [Crossref] [PubMed]
- Blaiss MS, Castro M, Chipps BE, et al. Guiding principles for use of newer biologics and bronchial thermoplasty for patients with severe asthma. Ann Allergy Asthma Immunol 2017;119:533-40. [Crossref] [PubMed]
- Beasley R. The burden of asthma with specific reference to the United States. J Allergy Clin Immunol 2002;109:S482-9. [Crossref] [PubMed]
- Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010;181:116-24. [Crossref] [PubMed]
- Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: a comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J 2017;50: [Crossref] [PubMed]
- British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline. 2016. Available online: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/. Accessed January 30, 2018.
- 2018 GINA Report, Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007;120:S94-138. [Crossref] [PubMed]
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73. [Crossref] [PubMed]
- d'Hooghe JNS, Ten Hacken NHT, Weersink EJM, et al. Emerging understanding of the mechanism of action of Bronchial Thermoplasty in asthma. Pharmacol Ther 2018;181:101-7. [Crossref] [PubMed]
- Bergeron C, Boulet LP. Structural changes in airway diseases: characteristics, mechanisms, consequences, and pharmacologic modulation. Chest 2006;129:1068-87. [Crossref] [PubMed]
- Boulet LP. Airway remodeling in asthma: update on mechanisms and therapeutic approaches. Curr Opin Pulm Med 2018;24:56-62. [Crossref] [PubMed]
- Boulet LP, Laviolette M. Acute effects of bronchial thermoplasty: a matter of concern or an indicator of possible benefit to small airways? Eur Respir J 2017;49: [Crossref] [PubMed]
- Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 1985;2004:1946-53. [PubMed]
- Pretolani M, Bergqvist A, Thabut G, et al. Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: Clinical and histopathologic correlations. J Allergy Clin Immunol 2017;139:1176-85. [Crossref] [PubMed]
- Dyrda P, Tazzeo T, DoHarris L, et al. Acute response of airway muscle to extreme temperature includes disruption of actin-myosin interaction. Am J Respir Cell Mol Biol 2011;44:213-21. [Crossref] [PubMed]
- Pretolani M, Dombret MC, Thabut G, et al. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med 2014;190:1452-4. [Crossref] [PubMed]
- Debray MP, Dombret MC, Pretolani M, et al. Early computed tomography modifications following bronchial thermoplasty in patients with severe asthma. Eur Respir J 2017;49: [Crossref] [PubMed]
- Chakir J, Haj-Salem I, Gras D, et al. Effects of Bronchial Thermoplasty on Airway Smooth Muscle and Collagen Deposition in Asthma. Ann Am Thorac Soc 2015;12:1612-8. [PubMed]
- Hirst SJ. Regulation of airway smooth muscle cell immunomodulatory function: role in asthma. Respir Physiol Neurobiol 2003;137:309-26. [Crossref] [PubMed]
- Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol 2013;132:1295-302. [Crossref] [PubMed]
- American College of Chest Physicians. Position statement for coverage and payment for bronchial thermoplasty. 2014. Available online: http://www.chestnet.org/News/CHEST-News/2014/05/Position-Statement-for-Coverage-and-Payment-for-Bronchial-Thermoplasty. Accessed January 30, 2018.
- Weinstein AG. The potential of asthma adherence management to enhance asthma guidelines. Ann Allergy Asthma Immunol 2011;106:283-91. [Crossref] [PubMed]
- Boulet LP, Vervloet D, Magar Y, et al. Adherence: the goal to control asthma. Clin Chest Med 2012;33:405-17. [Crossref] [PubMed]
- Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnaires. Immunol Allergy Clin North Am 2005;25:107-30. [Crossref] [PubMed]
- Rand CS, Wise RA. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med 1994;149:S69-76; discussion S77-8.
- Lefebvre P, Duh MS, Lafeuille MH, et al. Burden of systemic glucocorticoid-related complications in severe asthma. Curr Med Res Opin 2017;33:57-65. [Crossref] [PubMed]
- Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol 2015;136:1488-95. [Crossref] [PubMed]
- Zeiger RS, Schatz M, Dalal AA, et al. Utilization and Costs of Severe Uncontrolled Asthma in a Managed-Care Setting. J Allergy Clin Immunol Pract 2016;4:120-9.e3. [Crossref] [PubMed]
- Zein JG, Menegay MC, Singer ME, et al. Cost effectiveness of bronchial thermoplasty in patients with severe uncontrolled asthma. J Asthma 2016;53:194-200. [Crossref] [PubMed]
- Zafari Z, Sadatsafavi M, Marra CA, et al. Cost-Effectiveness of Bronchial Thermoplasty, Omalizumab, and Standard Therapy for Moderate-to-Severe Allergic Asthma. PLoS One 2016;11:e0146003 [Crossref] [PubMed]
- Cangelosi MJ, Ortendahl JD, Meckley LM, et al. Cost-effectiveness of bronchial thermoplasty in commercially-insured patients with poorly controlled, severe, persistent asthma. Expert Rev Pharmacoecon Outcomes Res 2015;15:357-64. [Crossref] [PubMed]
- Menzella F, Zucchi L, Piro R, et al. A budget impact analysis of bronchial thermoplasty for severe asthma in clinical practice. Adv Ther 2014;31:751-61. [Crossref] [PubMed]
- Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010;181:315-23. [Crossref] [PubMed]
- Green RH, Brightling CE, Woltmann G, et al. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002;57:875-9. [Crossref] [PubMed]
- Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 2015;70:115-20. [Crossref] [PubMed]
- ten Brinke A, de Lange C, Zwinderman AH, et al. Sputum induction in severe asthma by a standardized protocol: predictors of excessive bronchoconstriction. Am J Respir Crit Care Med 2001;164:749-53. [Crossref] [PubMed]
- Chung KF. New treatments for severe treatment-resistant asthma: targeting the right patient. Lancet Respir Med 2013;1:639-52. [Crossref] [PubMed]
- Simpson JL, Scott R, Boyle MJ, et al. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 2006;11:54-61. [Crossref] [PubMed]
- Langton D, Sha J, Ing A, et al. Bronchial thermoplasty: activations predict response. Respir Res 2017;18:134. [Crossref] [PubMed]
- Krmisky W, Sobieszczyk MJ, Sarkar S. Thermal ablation for asthma: current status and technique. J Thorac Dis 2017;9:S104-9. [Crossref] [PubMed]
- d'Hooghe JN, Eberl S, Annema JT, et al. Propofol and Remifentanil Sedation for Bronchial Thermoplasty: A Prospective Cohort Trial. Respiration 2017;93:58-64. [Crossref] [PubMed]
- Saran JS, Kreso M, Khurana S, et al. Anesthetic Considerations for Patients Undergoing Bronchial Thermoplasty. Anesth Analg 2018;126:1575-9. [Crossref] [PubMed]
- Mayse ML, Laviolette M, Rubin AS, et al. Clinical Pearls for Bronchial Thermoplasty. J Bronchology Interv Pulmonol 2007;14:115-23.
- Svenningsen S, Eddy RL, Lim HF, et al. Sputum Eosinophilia and Magnetic Resonance Imaging Ventilation Heterogeneity in Severe Asthma. Am J Respir Crit Care Med 2018;197:876-84. [Crossref] [PubMed]
- Thomen RP, Sheshadri A, Quirk JD, et al. Regional ventilation changes in severe asthma after bronchial thermoplasty with (3)He MR imaging and CT. Radiology 2015;274:250-9. [Crossref] [PubMed]
- Trivedi A, Hall C, Hoffman EA, et al. Using imaging as a biomarker for asthma. J Allergy Clin Immunol 2017;139:1-10. [Crossref] [PubMed]
- Adams DC, Hariri LP, Miller AJ, et al. Birefringence microscopy platform for assessing airway smooth muscle structure and function in vivo. Sci Transl Med 2016;8:359ra131 [Crossref] [PubMed]
Cite this article as: Cárdenas-García J, Cheng G, Castro M. Bronchial thermoplasty: an update for the interventional pulmonologist. AME Med J 2018;3:82.