
Diagnosis and management of mesothelioma
Introduction
Mesothelioma is a rare, but deadly malignancy originating from mesothelial cells that line the body cavities. This disease is closely related to prior asbestos exposure with a latency period of several decades. Although mesothelioma can develop in other parts of the body such as the peritoneum, it most commonly involves the pleura and is largely considered a thoracic neoplasm. Most patients present with advanced disease, thus treatment is not commonly curative. Median overall survival ranges from 9 to 17 months irrespective of stage (1). Treatment options include chemotherapy, radiation, and surgery, and are based primarily on tumor stage and histology. Multimodal treatment has been shown to be the most effective approach, but only benefits select patients with favorable disease subtypes. Management should involve a multidisciplinary team, and in many instances the focus is on symptomatic control and palliation.
In this article, the authors review the diagnosis and treatment for patients with malignant pleural mesothelioma. An overview of the presentation, diagnostic tests, and pre-treatment evaluation will be presented. Disease management will be discussed with respect to the general approach and considerations, surgical techniques, and multimodal therapy. Treatment options for palliation of pain and dyspnea will also be reviewed.
Risk factors & epidemiology
Mesothelioma develops from the mesothelial cells that line body cavities. Most (81%) mesothelioma cases occur in the pleura, with fewer cases occurring in the peritoneum (9%) and rare cases involving the pericardium or testicle (2). The 5-year relative survival for mesothelioma patients diagnosed between the years 2007–2014 was only 9.1%, with 1-year survival of only 34.8–47.3% (3). The poor prognosis is in part related to the fact that this disease is often diagnosed at an advanced stage and is extremely difficult to treat.
The main risk factor for mesothelioma is asbestos exposure (4). There is typically a delay of 20 to 50 years between the exposure to asbestos and a mesothelioma diagnosis (5,6). It is estimated that asbestos will no longer be a factor in mesothelioma cases after the year 2042, but will be the likely cause in 34% of cases that occur between now and that year (7). Other environmental exposures, including some naturally occurring mineral fibers, are also thought to contribute to the occurrence of mesothelioma (8). Patients treated with radiation for lymphoma are also at higher risk for malignant mesothelioma (9). Smoking, however, is not a risk factor for mesothelioma (10).
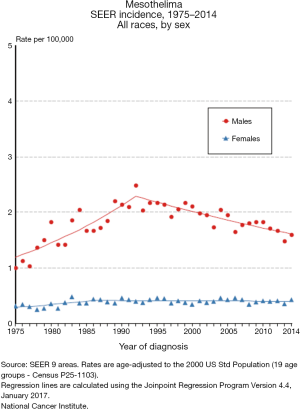
Mesothelioma is estimated to occur in 2,500 people in the United States each year, with an estimated prevalence count of 5,903 on January 1, 2014 (3,7). The incidence of mesothelioma increased slightly from 0.9 per 100,000 in the early 1980s to a peak of 1.2 per 100,000 in 1994–1995, with a subsequent decrease to 0.9 per 100,000 in 2012–2014 (3). The incidence rate for woman has been stable at 0.4 per 100,000 in the 1980s to the present, while the rate for men has decreased from a peak of 2.3 per 100,000 in 1992–1993 to 1.6 per 100,000 in 2012–2014 (Figure 1). Environmental and occupational exposures probably explain why mesothelioma rates are about four times higher in men compared to women, and about two times higher in whites compared to blacks. The decreased incidence in men over time is possibly due to reduced occupational exposure to substances such as asbestos. Mesothelioma is very uncommon in patients younger than 50, with median age at diagnosis of 62 and the majority of cases occurring in patients over the age of 65 (3,11). The highest incidence rates are observed in patients in their 70’s and 80’s (3).
The geographical distribution of 92,253 mesothelioma deaths reported by 83 countries to the World Health Organization in the years 1994–2008 was skewed towards high-income countries (12). The United States reported the highest number of mesothelioma deaths, and over 50% of all deaths occurred in Europe. Less than 12% were reported by middle- and low-income countries.
Pre-treatment assessment
Presentation
Patients with malignant pleural mesothelioma typically present with some combination of dyspnea, chest pain, cough, weight loss, fatigue, and pleural effusion (13). Diagnosis can be difficult given that these symptoms and clinical findings also typically occur in other much more common diseases, such as pneumonia (14). Suspicion of mesothelioma generally requires demographic factors or occupational or exposure history that are associated with mesothelioma.
Diagnosis
Workup based on the above symptoms often begins with a chest X-ray, which generally shows a large pleural effusion. Chest X-ray findings typically lead to a computed tomography (CT) scan, and thoracentesis is often performed to drain the effusion. Establishing a diagnosis of mesothelioma based on cytology can be difficult because the cytological findings of mesothelioma overlap with other benign conditions (15,16). Although several cytological and histological findings may raise varying levels of suspicion for mesothelioma, the current requirement for a definitive clinicopathological diagnosis is the demonstration of neoplastic invasion, such as infiltration into subpleural fat, chest wall skeletal muscle, rib or lung by histological examination. Diagnosis can also be made based on imaging studies and clinical exclusion of alternative causes for an atypical mesothelial proliferation (14,17,18). Since cytology does not provide assessment of tissue invasion, this test alone generally precludes the ability to make a diagnosis of mesothelioma.
Pleural biopsy is most often needed to demonstrate invasion and thus establish a diagnosis of mesothelioma. Options to obtain tissue include CT-guided core biopsy, video-assisted thoracoscopic (VATS) or open biopsy. Core biopsy with CT guidance can be a good option if there is an obvious mass present that is amenable to percutaneous biopsy (19). An advantage of surgical biopsy with VATS is that several areas can potentially be biopsied during the same procedure, and the use of frozen section can optimize the likelihood of obtaining a definitive diagnosis (20). In addition, any pleural effusion that is present can be drained, and chemical pleurodesis can be performed to manage the effusion. Open biopsy or thoracotomy should probably be restricted to a small incisional biopsy into the chest wall for those cases where the pleural space has been obliterated such that VATS is technically difficult to perform (14).
The histologic subtypes of mesothelioma include epithelioid, sarcomatoid (fibrous), and biphasic (mixed epithelioid and sarcomatoid) (21). Histology is a major factor with respect to both treatment and prognosis. Epithelioid is the most common histology (60%) and has the best prognosis, while sarcomatoid and biphasic are less common and have much worse prognoses (22,23). Establishing the histology prior to the initiation of treatment is critical in order to avoid unnecessary aggressive therapy, such as surgery in patients with sarcomatoid histology (24).
Pre-treatment evaluation
After a definitive diagnosis of mesothelioma has been made, subsequent evaluation is centered on establishing disease stage and assessing the patient’s suitability to tolerate treatment. Because symptoms often develop early on with local disease, patients typically present before the occurrence of distant metastasis (25). CT is the preferred modality for the initial radiological assessment (26). CT scans can often show multiple tumor characteristics as well as tumor extent, such as local invasion, lymph node involvement, and distant metastases. Quantitative FDG-PET parameters have prognostic and predictive significance in pleural mesothelioma (27,28). PET scans may show findings missed by CT, which is extremely important if radical surgery is being considered (29). It should be noted that the inflammatory process resulting from pleurodesis can lead to findings on CT, and especially PET, that could limit the assessment of local disease extent.
Staging with invasive procedures is not necessary in patients who are only planning to receive supportive care or palliative chemotherapy. However, invasive procedures may be required to confirm resectability if cancer-directly surgery is being considered. Extended staging with mediastinoscopy, endobronchial ultrasound, endoscopic ultrasound, VATS, or laparoscopy can all be utilized to confirm that the extent of disease will not preclude safe or attempted complete macroscopic resection (26,30-32). These procedures allow for the evaluation of contralateral disease, peritoneal disease, and lymph node involvement.
The staging system of mesothelioma is somewhat limited because large patient sets are not generally available to allow adequate development and validation of the prognostic importance of the staging elements. However, the American Joint Committee on Cancer (AJCC) TNM staging system is currently considered the best system for describing mesothelioma (33-36). The 8th edition of AJCC staging for mesothelioma is presented in Tables 1 and 2, although it should be noted that most studies and treatment guidelines reference prior editions of AJCC staging, which do not separate stage III into A and B subgroups. This revision was made primarily on the basis of revised nodal staging, where N1 and N2 in previous editions were combined into a single N1 category in the 8th edition, and N3 was reclassified as N2 (34). N1 disease now includes all ipsilateral intrathoracic lymph nodes while N2 includes contralateral and supraclavicular lymph nodes, allowing the distinction between locally advanced disease that is potentially resectable (IIIA) versus disease that is not (IIIB). When staging is assessed by surgery, the TNM disease stage is a significant predictor of prognosis (14). Unfortunately, CT and PET cannot always reliably determine T and N stage (37,38), however they are important tests for excluding metastatic disease in patients who are being considered for surgical treatment (28,29).
Table 1
| Category | Criteria |
|---|---|
| T category | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Ipsilateral parietal pleura, with or without visceral, mediastinal, diaphragmatic pleura involvement |
| T2 | All ipsilateral pleural surfaces (parietal, visceral, mediastinal, diaphragmatic), plus at least one: |
| ❖ Diaphragm muscle | |
| ❖ Pulmonary parenchyma | |
| T3 (potentially resectable) | All ipsilateral pleural surfaces (parietal, visceral, mediastinal, diaphragmatic), plus at least one: |
| ❖ Endothoracic fascia | |
| ❖ Mediastinal fat | |
| ❖ Solitary and completely resectable focus extending into chest wall soft tissue | |
| ❖ Pericardium, non-transmural | |
| T4 (unresectable) | All ipsilateral pleural surfaces (parietal, visceral, mediastinal, diaphragmatic), plus at least one: |
| ❖ Diffuse or multifocal chest wall | |
| ❖ Transdiaphragmatic extension | |
| ❖ Contralateral pleura | |
| ❖ Mediastinal organs | |
| ❖ Spine | |
| ❖ Pericardium, transmural | |
| N category | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Ipsilateral bronchopulmonary, hilar, mediastinal lymph nodes |
| N2 | Contralateral mediastinal or any supraclavicular lymph nodes |
| M category | |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
Table 2
| Stage | T | N | M |
|---|---|---|---|
| IA | T1 | N0 | M0 |
| IB | T2–T3 | N0 | M0 |
| II | T1–T2 | N1 | M0 |
| IIIA | T3 | N1 | M0 |
| IIIB | T1–T3 | N2 | M0 |
| T4 | Any N | M0 | |
| IV | Any T | Any N | M1 |
Additional tests to determine suitability for aggressive treatment should include pulmonary function testing and cardiac evaluation. For patients with diminished pulmonary function who are being assessed for extrapleural pneumonectomy (EPP), quantitative perfusion scanning can be done to further evaluate the ability to tolerate a pneumonectomy. Patients who have significantly compromised cardiac or pulmonary function may be better served by focusing on palliative treatments.
Management
Mesothelioma patients should be managed by a multidisciplinary team with expertise in treating this difficult disease (39). Curative treatment options include surgery, radiation, or chemotherapy. Management options also include supportive care and palliative chemotherapy. The most important characteristics that should be used to select treatment include disease stage, specific histology, and the ability to tolerate therapy, which is assessed by performance status and medical comorbidities. Consideration of surgery is recommended for medically fit patients with clinical stages I–III epithelioid mesothelioma based on prior AJCC staging systems (39), but should be restricted to stages I–IIIA disease according to AJCC 8th edition staging. For these patients, multimodality therapy is recommended. Chemotherapy alone is recommended for patients with stage IV disease or non-epithelioid histology and adequate performance status. Best supportive care should be utilized in patients with a performance status not adequate to tolerate chemotherapy.
Surgery
Surgical resection should only be undertaken for patients with stages I–III (or AJCC 8th edition stages I–IIIA) epithelioid mesothelioma who are medically fit. If EPP is being considered, adequate pulmonary reserve must be confirmed preoperatively with pulmonary function testing and, in some cases, quantitative perfusion imaging. The primary goal of surgical therapy is cytoreduction by achieving complete macroscopic resection. As described above, staging with invasive procedures may be necessary beforehand to assess for peritoneal, contralateral, or lymph node disease. During attempted resection, if complete macroscopic resection is not feasible surgery should be aborted.
Options for surgical resection of malignant pleural mesothelioma include EPP and pleurectomy/decortication (PD). EPP involves en bloc resection of the parietal pleura, lung, pericardium, and diaphragm followed by pericardial and diaphragmatic reconstruction, while PD is a lung-sparing technique resulting in complete removal of the entire ipsilateral pleural surfaces. An extended PD also involves pericardial and diaphragmatic resection and reconstruction, but with lung preservation. Although EPP is the established operation for this disease, there is growing evidence that PD may achieve comparable long-term outcomes with less morbidity. It should be noted that neither surgical approach is capable of achieving R0 resection due to the inevitability of residual microscopic disease (1,40,41).
EPP is the more aggressive operation and likely achieves a more complete R1 resection compared to PD, but at the cost of a higher postoperative complication rate (42,43). Thus, the surgical morbidity of EPP may eliminate any potential benefit of a better gross resection (44). Some data comparing EPP and PD suggests that survival may be better with PD (1,40,42,45), although these findings are difficult to generalize across such a heterogeneous disease entity. The most appropriate surgical procedure should be determined on an individual basis after considering tumor distribution, pulmonary function, and surgical expertise.
Intraoperative adjunct therapies have been studied in an attempt to improve local control and treat micrometastatic disease that technically cannot be resected (46). Heated intraoperative chemotherapy (HIOC) involves the delivery of heated chemotherapy agents directly into the pleural cavity during surgery. The potential advantages include improved drug delivery to residual tumor cells, particularly with hyperthermia, and lower toxicity compared to systemic chemotherapy. Hyperthermic pleural lavage with an iodine-based solution has been shown to cause cellular necrosis and an inflammatory reaction in in vitro studies, which may lead to an anti-tumor response (47). Several clinical studies demonstrate safety and potential benefit with this technique (48,49). Photodynamic therapy (PDT) is a light-based therapy involving administration of a photosensitizing agent followed by activation with a specific wavelength of light, which produces a highly reactive form of oxygen leading to tumor cell death. The combination of PDT with surgery may improve overall survival in mesothelioma patients (50).
Despite published treatment guidelines and the potential survival benefit of surgical resection, a recent population-based study found that cancer-directed surgery overall was used in only 37% of patients with AJCC 6th edition stages I–III epithelioid mesothelioma, with a median survival of 19 months (24). The reasons why surgery appears to be underutilized is this subset of patients is not entirely clear and should be studied further. The role of surgery in malignant pleural mesothelioma will continue to evolve as patient selection and surgical techniques improve.
Multimodal therapy
Chemotherapy can be used alone for patients with stage IV disease or non-epithelioid histology (51-54). Survival benefit has been demonstrated with cisplatin-based chemotherapy alone compared to supportive therapy in patients with stages III and IV disease, irrespective of disease histology (55). The most appropriate first line regimen is combined cisplatin/pemetrexed, though pemetrexed/carboplatin or gemcitabine/cisplatin can also be options (56-58). Radiation alone is not recommended for therapy, but can be used as part of a multimodality regimen or for focal palliation (39).
The benefit of combining surgery with other treatments has been increasingly studied (59-62). In a propensity-matched analysis, trimodality therapy for epithelioid patients with chemotherapy, radiation, and surgery improved median survival to 23.4 months compared to 14.5 months with surgery alone (63). The National Comprehensive Cancer Network (NCCN) guidelines therefore recommend multimodality treatment that includes surgery for medically operable patients with clinical stages I–III (or AJCC 8th edition stages I–IIIA) disease and epithelioid histology (64). Specifically, the recommended treatment includes a combination of chemotherapy and surgical resection via either PD or EPP, with radiation given after EPP and considered after PD.
A number of targeted agents are currently being studied in malignant pleural mesothelioma, however data thus far is limited and results have been modest (65,66). Several small series suggest some benefit to checkpoint inhibitors such as pembrolizumab and nivolumab (67,68). Trials are ongoing to determine the role of these agents in previously treated patients.
Palliative or supportive therapy
In asymptomatic patients who are not undergoing aggressive treatment, observation for signs and symptoms of disease progression is appropriate. When symptoms develop, palliative therapies are an essential aspect in the management of mesothelioma patients. Dyspnea and pain are among the most common symptoms (69), and there are treatment options for both. Pain can be due to pleural-based disease, chest wall invasion, or nerve involvement. Aside from standard medical therapies, such as anti-inflammatory medications, opioids, and agents for neuropathic pain, palliative radiation may be beneficial in appropriately selected patients (70,71). Radiation therapy may also be useful in patients with dyspnea, esophageal symptoms, and superior vena cava syndrome provided the target lesion is confined to a relatively limited field (72).
Dyspnea can be related to restrictive chest wall processes, involvement of pulmonary parenchyma, or malignant pleural effusions. The latter can be treated with percutaneous drainage initially, followed by a more durable treatment such as pleurodesis or a tunneled pleural catheter if symptoms improve with fluid removal. Anxiety may also manifest as dyspnea, and treatment with anxiolytics such as benzodiazepines can improve symptoms in these instances.
Summary
Malignant pleural mesothelioma remains a challenging disease to treat with an overall poor prognosis. Treatment goals for patients with advanced disease and unfavorable histology should be centered on providing palliation of symptoms and optimizing quality of life. For select patients with epithelioid histology and potentially resectable disease who are medically fit, aggressive multimodal therapy including surgery offers the best long-term survival. PD is a lung-sparing surgical option that is less morbid and yields similar or improved outcomes compared to EPP, particularly when combined with other treatment modalities. Ongoing research in adjunct intraoperative therapies and targeted agents will hopefully improve the outlook for this formidable disease in the near future.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Amit Mahajan, Sandeep J. Khandhar and Erik E. Folch) for the series “Management of Complex Airway and Pleural Diseases” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.09.11). The series “Management of Complex Airway and Pleural Diseases” was commissioned by the editorial office without any funding or sponsorship. Mark F. Berry serves as an unpaid editorial board member of AME Medical Journal from Aug 2016 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsao AS, Wistuba I, Roth JA, et al. Malignant pleural mesothelioma. J Clin Oncol 2009;27:2081-90. [Crossref] [PubMed]
- Special Section: Rare Cancers in Adults. American Cancer Society. Cancer Facts & Figures 2017.
- Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER website, April 2017.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite and anthophyllite). A review of human carcinogens Part C: Arsenic, metals, fibres and dusts. Lyon, France: International Agency for Research on Cancer, 2012.
- Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med 1992;34:718-21. [PubMed]
- Selikoff IJ, Hammond EC, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 1980;46:2736-40. [Crossref] [PubMed]
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39:576-88. [Crossref] [PubMed]
- Baumann F, Carbone M. Environmental risk of mesothelioma in the United States: An emerging concern-epidemiological issues. J Toxicol Environ Health B Crit Rev 2016;19:231-49. [Crossref] [PubMed]
- Chang ET, Lau EC, Mowat FS, et al. Therapeutic radiation for lymphoma and risk of second primary malignant mesothelioma. Cancer Causes Control 2017;28:971-9. [Crossref] [PubMed]
- Mossman BT, Lippmann M, Hesterberg TW, et al. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J Toxicol Environ Health B Crit Rev 2011;14:76-121. [Crossref] [PubMed]
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of Survival in Malignant Pleural Mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) Study of 14,228 Patients. PLoS One 2015;10:e0145039 [Crossref] [PubMed]
- Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011;89:716-24, 724A-C.
- Yates DH, Corrin B, Stidolph PN, et al. Malignant mesothelioma in south east England: Clinicopathological experience of 272 cases. Thorax 1997;52:507-12. [Crossref] [PubMed]
- van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254-307. [PubMed]
- Henderson DW, Shilkin KB, Whitaker D. Reactive mesothelial hyperplasia vs mesothelioma, including mesothelioma in situ: a brief review. Am J Clin Pathol 1998;110:397-404. [Crossref] [PubMed]
- Churg A, Colby TV, Cagle P, et al. The separation of benign and malignant mesothelial proliferations. Am J Surg Pathol 2000;24:1183-200. [Crossref] [PubMed]
- British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax 2007;62:ii1-19. [PubMed]
- Husain AN, Colby TV, Ordonez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: A consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2009;133:1317-31. [PubMed]
- Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. [Crossref] [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Husain AN, Colby TV, Ordóñez NGGuidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group, et al. Arch Pathol Lab Med 2018;142:89-108. [Crossref] [PubMed]
- Edwards JG, Abrams KR, Leverment JN, et al. Prognostic factors for malignant mesothelioma in 142 patients: Validation of CALGB and EORTC prognostic scoring systems. Thorax 2000;55:731-5. [Crossref] [PubMed]
- Neumann V, Rutten A, Scharmach M, et al. Factors influencing long-term survival in mesothelioma patients--results of the German mesothelioma register. Int Arch Occup Environ Health 2004;77:191-9. [Crossref] [PubMed]
- Meyerhoff RR, Yang CF, Speicher PJ, et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res 2015;196:23-32. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Schouwink JH, Kool LS, Rutgers EJ, et al. The value of chest computer tomography and cervical mediastinoscopy in the preoperative assessment of patients with malignant pleural mesothelioma. Ann Thorac Surg 2003;75:1715-8. [Crossref] [PubMed]
- Basu S, Saboury B, Torigian DA, et al. Current Evidence Base of FDG-PET/CT Imaging in the Clinical Management of Malignant Pleural Mesothelioma: Emerging Significance of Image Segmentation and Global Disease Assessment. Mol Imaging Biol 2011;13:801-11. [Crossref] [PubMed]
- Wilcox BE, Subramaniam RM, Peller PJ, et al. Utility of integrated computed tomography-positron emission tomography for selection of operable malignant pleural mesothelioma. Clin Lung Cancer 2009;10:244-8. [Crossref] [PubMed]
- Erasmus JJ, Truong MT, Smythe WR, et al. Integrated computed tomography-positron emission tomography in patients with potentially resectable malignant pleural mesothelioma: Staging implications. J Thorac Cardiovasc Surg 2005;129:1364-70. [Crossref] [PubMed]
- Alvarez JM, Hasani A, Segal A, et al. Bilateral thoracoscopy, mediastinoscopy and laparoscopy, in addition to CT, MRI and PET imaging, are essential to correctly stage and treat patients with mesothelioma prior to trimodality therapy. ANZ J Surg 2009;79:734-8. [Crossref] [PubMed]
- Rice DC, Erasmus JJ, Stevens CW, et al. Extended surgical staging for potentially resectable malignant pleural mesothelioma. Ann Thorac Surg 2005;80:1988-92. [Crossref] [PubMed]
- Tournoy KG, Burgers SA, Annema JT, et al. Transesophageal endoscopic ultrasound with fine needle aspiration in the preoperative staging of malignant pleural mesothelioma. Clin Cancer Res 2008;14:6259-63. [Crossref] [PubMed]
- Nowak AK, Chansky K, Rice DC, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2089-99.
- Rice D, Chansky K, Nowak A, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2100-11.
- Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol 2016;11:2112-9. [Crossref] [PubMed]
- Amin MB, Greene FL, Byrd DR. AJCC Cancer Staging Manual, 8th edition. Springer International Publishing, 2017:1-1024.
- Flores RM, Akhurst T, Gonen M, et al. Positron emission tomography defines metastatic disease but not locoregional disease in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2003;126:11-6. [Crossref] [PubMed]
- Pilling J, Dartnell JA, Lang-Lazdunski L. Integrated positron emission tomography-computed tomography does not accurately stage intrathoracic disease of patients undergoing trimodality therapy for malignant pleural mesothelioma. Thorac Cardiovasc Surg 2010;58:215-9. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Borghaei H, et al. Malignant pleural mesothelioma. J Natl Compr Canc Netw 2012;10:26-41. [Crossref] [PubMed]
- Friedberg JS. The state of the art in the technical performance of lung-sparing operations for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2013;25:125-43. [Crossref] [PubMed]
- Hasani A, Alvarez JM, Wyatt JM, et al. Outcome for patients with malignant pleural mesothelioma referred for Trimodality therapy in Western Australia. J Thorac Oncol 2009;4:1010-6. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Schipper PH, Nichols FC, Thomse KM, et al. Malignant pleural mesothelioma: surgical management in 285 patients. Ann Thorac Surg 2008;85:257-64. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6. [Crossref] [PubMed]
- Chan WH, Sugarbaker DJ, Burt BM. Intraoperative adjuncts for malignant pleural mesothelioma. Transl Lung Cancer Res 2017;6:285-94. P. [Crossref] [PubMed]
- Fiorelli A, Pentimalli F, D'Urso V, et al. Antineoplastic activity of povidone-iodine on different mesothelioma cell lines: results of in vitro study. Eur J Cardiothorac Surg 2014;45:993-1000. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Papa S, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and systemic chemotherapy in patients with malignant pleural mesothelioma: a 10-year experience. J Thorac Cardiovasc Surg 2015;149:558-65; discussion 565-6. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Belcher E, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine followed by adjuvant chemotherapy in patients with malignant pleural mesothelioma. J Thorac Oncol 2011;6:1746-52. [Crossref] [PubMed]
- Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg 2012;93:1658-65; discussion 1665-7.
- Bovolato P, Casadio C, Billè A, et al. Does surgery improve survival of patients with malignant pleural mesothelioma?: a multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol 2014;9:390-6. [Crossref] [PubMed]
- Blomberg C, Nilsson J, Holgersson G, et al. Randomized Trials of Systemic Medically-treated Malignant Mesothelioma: A Systematic Review. Anticancer Res 2015;35:2493-501. [PubMed]
- Kelly RJ, Sharon E, Hassan R. Chemotherapy and targeted therapies for unresectable malignant mesothelioma. Lung Cancer 2011;73:256-63. [Crossref] [PubMed]
- Ellis P, Davies AM, Evans WK, et al. The use of chemotherapy in patients with advanced malignant pleural mesothelioma: a systematic review and practice guideline. J Thorac Oncol 2006;1:591-601. [Crossref] [PubMed]
- Metintas M, Ak G, Erginel S, et al. A retrospective analysis of malignant pleural mesothelioma patients treated either with chemotherapy or best supportive care between 1990 and 2005 A single institution experience. Lung Cancer 2007;55:379-87. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Castagneto B, Botta M, Aitini E, et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 2008;19:370-3. [Crossref] [PubMed]
- Arrieta O, López-Macías D, Mendoza-García VO, et al. A phase II trial of prolonged, continuous infusion of low-dose gemcitabine plus cisplatin in patients with advanced malignant pleural mesothelioma. Cancer Chemother Pharmacol 2014;73:975-82. [Crossref] [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [Crossref] [PubMed]
- Thieke C, Nicolay NH, Sterzing F, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol 2015;10:267. [Crossref] [PubMed]
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8. [Crossref] [PubMed]
- Kapeles M, Gensheimer MF, Mart DA, et al. Trimodality Treatment of Malignant Pleural Mesothelioma: An Institutional Review. Am J Clin Oncol 2018;41:30-35. [PubMed]
- Nelson DB, Rice DC, Niu J, et al. Long-Term Survival Outcomes of Cancer-Directed Surgery for Malignant Pleural Mesothelioma: Propensity Score Matching Analysis. J Clin Oncol 2017;35:3354-62. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Malignant Pleural Mesothelioma, Version 3.2016. J Natl Compr Canc Netw 2016;14:825-36. [Crossref] [PubMed]
- Stahel RA, Weder W, Felley-Bosco E, et al. Searching for targets for the systemic therapy of mesothelioma. Ann Oncol 2015;26:1649-60. [Crossref] [PubMed]
- Bronte G, Incorvaia L, Rizzo S, et al. The resistance related to targeted therapy in malignant pleural mesothelioma: Why has not the target been hit yet? Crit Rev Oncol Hematol 2016;107:20-32. [Crossref] [PubMed]
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
- Quispel-Janssen J, van der Noort V, de Vries JF, et al. Programmed Death 1 Blockade With Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma. J Thorac Oncol 2018;13:1569-76. [Crossref] [PubMed]
- Abrahm JL. Palliative care for the patient with mesothelioma. Semin Thorac Cardiovasc Surg 2009;21:164-71. [Crossref] [PubMed]
- van Thiel ER, Surmont VF, van Meerbeeck JP. Malignant pleural mesothelioma: when is radiation therapy indicated? Expert Rev Anticancer Ther 2011;11:551-60. [Crossref] [PubMed]
- Jenkins P, Milliner R, Salmon C. Re-evaluating the role of palliative radiotherapy in malignant pleural mesothelioma. Eur J Cancer 2011;47:2143-9. [Crossref] [PubMed]
- Baldini EH. Radiation therapy options for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2009;21:159-63. [Crossref] [PubMed]
Cite this article as: Liou DZ, Berry MF. Diagnosis and management of mesothelioma. AME Med J 2018;3:99.

