
Oncogenic potential of BMI1: race-based evidence in prostate cancer
A recent publication by Ganaie et al. (1) in Clinical Cancer Research (doi: 10.1158/1078-0432.CCR-18-1394, 2018) demonstrate BMI1 as a potential driver of metastasis in prostate cancer. The authors validate the significance of BMI1 in prostate cancer metastasis in African-American and Caucasian-American patients. Alongside the authors examine the efficacy of small molecule inhibitor of BMI1 (PTC-209) against localized and metastatic models of African-American and Caucasian disease in vitro models employing various race-specific prostate cell lines and in vivo mouse xenograft model and zebrafish. This published study has immense clinical implication proposing BMI1 as a promising therapeutic target for the treatment of advance-stage prostate cancer including those categorized as high-risk groups.
B cell-specific Moloney murine leukemia virus integration site 1 (BMI1) is a polycomb repressive complex 1 (PRC1) member also known as polycomb group RING finger protein 4 (PCGF4) or RING finger protein 51 (RNF51) that plays a critical role in cancer progression (2). As name implies, the gene encrypting BMI was first identified as an oncogene inducing B- and T-cell leukemia’s in stem cells that ascertains the self-renewal and proliferative capabilities of normal and leukemic stem cells (3). BMI1 gene is 10.04 kb in size with 10 exon having highly conserved sequence between various species (4). The human BMI1 gene is localized on chromosome 10 (10p11.23) (Figure 1A). The BMI1 protein comprises of 326 amino acids having a molecular weight of 36.95 kDa with a RING finger at the N-terminus and a central helix-turn-helix domain (Figure 1B). The ring finger domain is a cysteine rich domain (CRD) which functions in zinc binding and contributes to the ubiquitination process. Binding of BMI1 to Ring 1B initiates the E3 ubiquitin ligase activity together with the RING domain and the extended N-terminal tail facilitates to the interaction of BMI1 and Ring 1B (5).
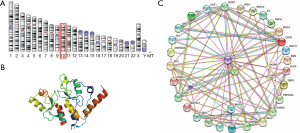
BMI1 is the essential constituent of the polycomb group complex 1 (PRC1) playing an important role in the regulation of H2A ubiquitination activity and facilitate protein-protein interaction (6). The polycomb group of proteins (PcG) classified as PRC1 and PRC2 complex are essential in inducing cell proliferation, differentiation, maintenance stem cell renewal, and regulation of cellular memories (7). These molecules employ their function by establishing multimeric chromatin-associated protein complexes and suppressing downstream targets causing gene silencing (6,7). The PRC1 complex is composed of four core subunits: CBX (polycomb; CBX2/4/6/7/8), either B lymphoma Mo-MLV insertion region 1 or PCGF (polycomb group factors; PCGF1–6), PHC (polyhomeotic homologues; PHC1/2/3) and RING E3 ligase (RING1A/B) (8). Evidences suggests the presence of a central domain for BMI1 is critical for being involved in protein-protein interactions and oncogenic activity (9).
Numerous studies have documented BMI1 as a fundamental regulator of several cellular processes including self-renewal of stem cells and cancer cell proliferation mainly targeting repression of CDKN2a locus that encodes two structurally diverse proteins, p16/INK4a and p19/ARF, both of which control cellular proliferation in response to mitogenic signaling (8). BMI1 interacts with several molecules of the Wnt/β-catenin, PI3K/Akt, Notch, Hedgehog signaling pathways and some receptor tyrosine kinases (Figure 1C). Knockdown of BMI1 gene significantly alters the Notch and Wnt signaling pathways which are critical in the development of Ewing sarcoma family of tumors (10). BMI1 has an effect on the Hedgehog signaling pathway regulating mammary stem cell proliferation (11). BMI1 is also regarded as a basic factor in controlling Th2 cell differentiation and development through stabilization of GATA transcription factors (12). Aberrant BMI1 expression has been identified in various human cancers including bladder, skin, prostate, breast, colorectal, ovarian, and in some hematological malignancies (13). BMI1 is known to be involved in the epigenetic regulation of cancer cell proliferation, cell invasion, metastasis, chemo-sensitivity and patient survival (14). Silencing of BMI1 causes impairment of cancer cell proliferation and tumor growth in various tumor models (15). These results suggest that BMI1 has oncogenic potential and could be developed as a valid therapeutic target.
In the prostate, BMI1 is in abundance in prostatic luminal cells and its higher levels correlates with poor prognosis of prostate cancer patients (16,17). Patients with tumors of Gleason score 8 or higher exhibit significant upregulation of BMI1, while its presence in low-grade prostate cancer specimens remains predictive for prostate-specific antigen (PSA) recurrence (17). Glinsky et al. [2005] demonstrated by microarray meta-analyses that presence of BMI1 in prostate cancer specimens correlates with metastatic progression with higher probability of unfavorable therapeutic outcome (18). In a population of prostate cancer cells, BMI1 enrichment leads to higher tumor initiating capacity (19). Despite these findings the evidence for functional involvement of BMI1 in prostate cancer progression is lacking.
Ganaie et al. compared the expression of BMI1 in metastatic tumors from Caucasian-Americans and African-American patients further strengthening its relevance. The authors demonstrate that BMI1 expression is higher in African-American primary tumors compared to grade-matched Caucasian patients. Additional determination of BMI1 expression in matched normal and tumor tissues of African-American patients using qPCR and immunohistochemistry exhibit significant increase in its expression. The study further validated the enrichment of BMI1 in prostate tumors from African-American by applying bioinformatics and data mining. Previous reports suggest that BMI1 does not only drive human mammary epithelial cells to bypass senescence and immortalization, but can also play a key role in breast cancer progression (20).
The authors make significant observations in the study by determining the expression of several genes associated to BMI1 in metastatic prostate tumors of African-American and Caucasian-Americans. The findings suggest that BMI1 enriched metastatic tumors exhibit elevated expression of c-Myc, VEGF, CyclinD1 and MMP-2. The highlight of this study is the race-specific comparison showing higher levels of BMI1 expression and its regulators in African-American prostate tumors. Furthermore, the study demonstrated decreased expression of two major tumor suppressors PTEN and p16/INK4a. Though the finding is global the authors underline the importance of decreased expression of p16/INK4a in the African-American metastatic tumors whereas diminished expression of PTEN was observed in both races. BMI1 has been reported to directly repress p16/INK4a while PTEN inhibits BMI1 function independent of its phosphatase activity (21). A recent study by Zhu et al. [2018] demonstrates that BMI1 regulates androgen receptor independent of PRC1 complex facilitating development of castration-resistant prostate cancer and resistance to enzalutamide treatment (22). As a highlight the authors demonstrate that BMI1 is recruited to the p16/INK4a and PTEN promoter to cause their repression specifically in African–American primary cancer cell lines. In addition, the authors silenced BMI1 in metastatic prostate cancer cells demonstrating a concomitant decrease in the expression of VEGF, c-Myc and MMP-2 as an evidence for the oncogenic potential of BMI1 facilitating prostate cancer metastasis.
Kreso et al. [2014] developed a small-molecule BMI1 inhibitor PTC-209 having precise antitumor activity in colorectal cancer. PTC-209 exposure to colorectal cancer cells reduces cell growth and stem cell properties (23). The authors employ PTC-209 in African-American and Caucasian models to show its effect on the inhibition of BMI1 expression, promoter activity and regulation of target genes suppressing metastatic growth of African-American tumor cells. Overall, the authors have demonstrated the effect of BMI1 inhibition through genetic manipulation and pharmacological inhibition suggesting it as a druggable target in the treatment of metastatic prostate cancer. The authors also validated the anti-metastatic potential of PTC-209 in two in vivo models, Zebrafish and nude mouse xenograft. Treatment with PTC-209 caused significant reduction in metastasis in both the models. PTC-209 also caused reduction in extravasation and cell migration in the zebrafish and significantly reduced the number of micro-metastasis nodules in the nude mice xenograft.
Several studies have established that difference in ethnic and/or genetic background could contribute to the disparity in the therapeutic outcome of prostate cancer (24). In this study, the authors focus on understanding the mechanism which underlies the metastasis and therapeutic outcome in prostate cancer patients, particularly considering race as a determinant factor. Docetaxel remains the first-line treatment modality for metastatic castrate-resistant prostate cancer. ECOG-led phase III randomized trial conducted in prostate cancer patients (89% Caucasian) suggested that docetaxel performs better under combination settings with androgen deprivation therapy (ADT) (25). Since African-American exhibit poor prognosis compared to Caucasians, however ADT+ docetaxel combination therapy produced comparable outcome in patients of both races. In similar context applying the concept of combinatorial therapy, the authors investigate the efficacy of combining BMI1 inhibitor with docetaxel utilizing in vitro and in vivo models of prostate cancer. The results demonstrate that individual treatment with docetaxel to African-American prostate tumor models did not alter the expression of BMI1 or its regulatory genes; whereas combination with PTC-209 reduced the expression of BMI1 and its regulated genes suppressing proliferation and improving responsiveness to docetaxel therapy. Thus, BMI1-targeting could be envisaged as a strategy to improve the clinical outcome of docetaxel in metastatic disease.
The significance of this manuscript lies in the concept to demonstrate BMI1 possessing oncogenic potential and driver of prostate cancer metastasis. The authors also provide adequate evidence to demonstrate that BMI1 expression is significantly higher in African-American prostate cancer patients. Taken together, the study is of high clinical impact in suggesting BMI1-targeted therapy could be developed as an adjuvant complementing docetaxel therapy to effectively treat metastatic disease in patients harboring untreatable metastatic tumors across ethnic backgrounds.
Acknowledgements
Funding: Efforts are supported by VA Merit Review 1I01BX002494 and the Department of Defense Grants W81XWH-18-1-0618 and W81XWH-15-1-0558 to S Gupta.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiao Li (Department of Urologic Surgery, the Affiliated Cancer Hospital of Jiangsu Province of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.11.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ganaie AA, Beigh FH, Astone M, et al. BMI1 Drives Metastasis of Prostate Cancer in Caucasian and African-American Men and Is A Potential Therapeutic Target: Hypothesis Tested in Race-specific Models. Clin Cancer Res 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Goebl MG. The bmi-1 and mel-18 gene products define a new family of DNA-binding proteins involved in cell proliferation and tumorigenesis. Cell 1991;66:623. [Crossref] [PubMed]
- Alkema MJ, Jacobs H, van Lohuizen M, et al. Pertubation of B and T cell development and predisposition to lymphomagenesis in Emu Bmi1 transgenic mice require the Bmi1 RING finger. Oncogene 1997;15:899-910. [Crossref] [PubMed]
- Itahana K, Zou Y, Itahana Y, et al. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol 2003;23:389-401. [Crossref] [PubMed]
- Li Z, Cao R, Wang M, et al. Structure of a Bmi-1-Ring1B polycomb group ubiquitin ligase complex. J Biol Chem 2006;281:20643-9. [Crossref] [PubMed]
- Wang H, Wang L, Erdjument-Bromage H, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature 2004;431:873-8. [Crossref] [PubMed]
- Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest 2004;113:175-9. [Crossref] [PubMed]
- Gray F, Cho HJ, Shukla S, et al. BMI1 regulates PRC1 architecture and activity through homo- and hetero-oligomerization. Nat Commun 2016;7:13343. [Crossref] [PubMed]
- Jacobs JJ, Kieboom K, Marino S, et al. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 1999;397:164-8. [Crossref] [PubMed]
- Douglas D, Hsu JH, Hung L, et al. BMI-1 promotes ewing sarcoma tumorigenicity independent of CDKN2A repression. Cancer Res 2008;68:6507-15. [Crossref] [PubMed]
- Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 2006;66:6063-71. [Crossref] [PubMed]
- Hosokawa H, Kimura MY, Shinnakasu R, et al. Regulation of Th2 cell development by Polycomb group gene bmi-1 through the stabilization of GATA3. J Immunol 2006;177:7656-64. [Crossref] [PubMed]
- Glinsky GV. "Stemness" genomics law governs clinical behavior of human cancer: implications for decision making in disease management. J Clin Oncol 2008;26:2846-53. [Crossref] [PubMed]
- Marquardt JU, Factor VM, Thorgeirsson SS. Epigenetic regulation of cancer stem cells in liver cancer: current concepts and clinical implications. J Hepatol 2010;53:568-77. [Crossref] [PubMed]
- Liu Y, Liu F, Yu H, et al. Akt phosphorylates the transcriptional repressor bmi1 to block its effects on the tumor-suppressing ink4a-arf locus. Sci Signal 2012;5:ra77. [Crossref] [PubMed]
- Siddique HR, Parray A, Zhong W, et al. BMI1, stem cell factor acting as novel serum-biomarker for Caucasian and African-American prostate cancer. PLoS One 2013;8:e52993 [Crossref] [PubMed]
- van Leenders GJ, Dukers D, Hessels D, et al. Polycomb-group oncogenes EZH2, BMI1, and RING1 are overexpressed in prostate cancer with adverse pathologic and clinical features. Eur Urol 2007;52:455-63. [Crossref] [PubMed]
- Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest 2005;115:1503-21. [Crossref] [PubMed]
- Hurt EM, Kawasaki BT, Klarmann GJ, et al. CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer 2008;98:756-65. [Crossref] [PubMed]
- Dimri GP, Martinez JL, Jacobs JJ, et al. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res 2002;62:4736-45. [PubMed]
- Fan C, He L, Kapoor A, et al. PTEN inhibits BMI1 function independently of its phosphatase activity. Mol Cancer 2009;8:98. [Crossref] [PubMed]
- Zhu S, Zhao D, Yan L, et al. BMI1 regulates androgen receptor in prostate cancer independently of the polycomb repressive complex 1. Nat Commun 2018;9:500. [Crossref] [PubMed]
- Kreso A, van Galen P, Pedley NM, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med 2014;20:29-36. [Crossref] [PubMed]
- Whittemore AS, Kolonel LN, Wu AH, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst 1995;87:652-61. [Crossref] [PubMed]
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol 2018;36:1080-7. [Crossref] [PubMed]
Cite this article as: Shankar E, Verma S, Gupta S. Oncogenic potential of BMI1: race-based evidence in prostate cancer. AME Med J 2018;3:108.

