
Malignant pleural effusion and mesothelioma
Malignant pleural mesothelioma (MPM) still represents a major clinical challenge, with most of the main aspects being sufficiently covered by several existing guidelines (BTS, ERS/EACTS etc.). These guidelines gave quite clear diagnostic algorithms and evidence-based recommendations for treatment options, with advantages and draw-backs for each approach. That is why the subject of this chapter are only specificities of the mesothelioma-related pleural effusion (PE): what are the differences vs. other malignant pleural effusions (MPEs), what is the reliability of non-invasive and cytological diagnostics, what is its role in follow up and what is the optimal pleural fluid management. In the era of the widespread use of VATS procedures, enabling precise biopsy of any underlying lesion under the eye control, it is clear that the practical significance of MPM-associated PE itself relates mostly to patients being unfit for medical or surgical thoracoscopic biopsies.
Differences between MPM-related PE and other MPE
From the clinical standpoint, the main difference relates to the need for further invasive diagnostics after initial cytological analysis. Unlike patients with PE accompanying primary or secondary lung or mediastinal tumours, in whom pleural fluid cytology may be often enough for diagnosis, in case of MPM-associated effusion, the need for thoracoscopic/VATS biopsy is much more frequent. This because in a quite high proportion of patients with lung cancer, owing to typical radiographic aspect, the histological diagnosis of the underlying malignancy is usually obtained by bronchoscopy, mediastinoscopy or transcutaneous needle biopsy, thus obviating the need for further pleural biopsies to confirm the cause of the PE. In most of these patients, the accompanying pleural fluid frequently means the advanced disease, eliminating eventual surgical treatment, thus switching the focus of PE management to the type of pleurodesis as a part of palliative treatment. Unlike that, in patients with MPM, especially in those without the lung and/or mediastinal lesions, the diagnostic possibilities are limited to the region of the pleura and importantly, the accompanying PE itself, independently of size and aspect, does not preclude eventual surgery. In addition, the high operative Mb and Mt require biopsies with sufficient volume of tissue to establish the reliable diagnosis before offering surgery to the patient. Secondly, PE may be present only in the early disease stage, followed by complete resolution synchronously with disease progression in form of the circumferential tumour growth from the parietal pleura. Such a scenario additionally complicates the diagnostics. As the volume of the PE is not of great help in practice, patients with previous asbestos exposure and PE, even if small, should be carefully monitored, because in some of them MPM will develop (1).
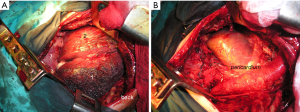
The radiographic aspect of PE does not correlate neither with the mesothelioma growth pattern nor with the disease extent. Two different morphological patterns of mesothelioma, each with PE as initial manifestation, in patients who underwent surgery are presented on Figures 1 and 2.

Value of noninvasive diagnostics [computed tomography (CT), PET, magnetic resonance imaging (MRI)]
In patients with any PE, the standard chest radiography can serve only for initial orientation. In the great majority of them some additional imaging is necessary.
One of the most important practical benefits of CT in patients with MPM, especially in presence of small amount of fluid and/or suspected major pleural adhaesions, is to identify eventual areas on the parietal pleura that are accessible by transcutaneous needle-biopsy. This is also very important in patients unsuitable for thoracoscopy for any reason.
The diagnostic accuracy of CT in patients with a malignant pleural dissemination/involvement ranges between 70% and 97%. The differentiation between MPM and other pleural malignancies is difficult. Features favoring MPM over metastatic pleural malignancy are fissure involvement and the absence of the lung lesions (2). The presence of pleural plaques per se is not a reliable sign of malignancy, because the PE may also exist due to benign asbestos-related PE.
The accuracy of CT is quite low for the assessment of invasion of the chest wall and diaphragm. The CT is not so accurate for mediastinal staging, so that more than a third of patients are subsequently upstaged after a PET scan (3).
The use of PET-CT can help to determine a nature of some findings that remain unclear after CT and to better determine the areas for biopsies. It should be mentioned that, even under the thoracoscopic view, it is not always easy to distinguish between sequellar or constitutional lesions and tumour growth. Areas of pleural thickenings containing a malignant tissue have elevated maximal standardised uptake values (SUVmax) (4,5). A SUVmax threshold of >2.0 was demonstrated as reliable to differentiate between malignant and nonmalignant pleural lesions with sensitivity and specificity being 88–100% and 88–92%, respectively (6-8). The false negative results may be due to small tumour diameter and low proliferative index, like in early stage epithelioid mesothelioma. False positive results may be mostly the consequence of different inflammations, tuberculosis, or talc pleurodesis.
In brief, despite the role in identifying targets for biopsies, PET-CT alone cannot differentiate MPM from pleural carcinosis and no causative relationship between SUVmax and histological sub-type could be demonstrated (6). In addition, poor spatial resolution of PET-CT, results in low sensitivity for extrapleural invasion.
Despite the limited role of MRI in MPM staging, MRI is superior to CT in detecting invasion of the chest wall, diaphragmatic muscle and mediastinal fat, thus significantly improving the overall diagnostic accuracy (9).
More recently, the diffusion-weighted MRI (DWI-MRI) appeared as potentially useful, in a way that lower values of apparent diffusion coefficient (ADC) exist in pleural malignancy, compared with benign disease (10). That is a consequence of the thickened pleura and may be of help in practice (92.5% sensitivity, 79% specificity (11). It was also demonstrated that epithelioid type of mesothelioma had a significantly higher ADC value than non-epithelioid, with the suggested threshold of 1.1 (sensitivity of 60% and specificity of 94%) (12). The latter finding could be also useful in practice, but these MRI data still need to be prospectively validated.
Role of cytological diagnostics
Although some laboratories are reliable for definitive cytological diagnosis, the International Mesothelioma Interest Group recommends biopsy to establish the definitive diagnosis (13). So, what are the limitations of cytological analysis of pleural fluid in suspected MPM?
First, many currently performed testes can be done on both pleural fluid and biopsy samples, but the sensitivity is reduced if no atypical mesothelial cells are present in pleural fluid (14,15). Although the clinical value of several markers has been analysed, it was limited for many of them, because sarcomatoid type usually does not express typical ‘mesothelial’ markers. That is why the use of keratins (quite non-specific as a marker), still remains appropriate.
Second, it is difficult to distinguish between sarcomatoid type of mesothelioma and other sarcomatous tumours. The use of GATA3 was reported with promising results (16). Analysis of BAP1 expression (if absent) and of p16 deletion by using the FISH method (fluorescent in-situ hybridization) may suggest the presence of MPM, but are not specific (17,18).
Positive immunohistochemical markers for MPM are calretinin, thrombomodulin, CK5/6, CAM5.2, EMA, vimentin, GLUT-1, HBME-1, WT-1, p53, with 45–100% sensitivity. Negative markers are Ber-Ep4, MOC-31, CEA, Leu-1, CD15, TTF-1, B72.3, with 53–100% overall specificity. By combining two positive mesothelioma and two negative adenocarcinoma markers may increases the diagnostic accuracy according to BTS guidelines (19).
The over-expression of mesothelin also exists in patients with MPM. The values of serum mesothelin increase in patients with MPM compared to control group of asbestos-exposed patients (20,21). A meta-analysis on 460 MPM and 1,046 non-MPM demonstrated the overall sensitivity and specificity of pleural fluid SMRP of 75%, and 76%, respectively (22). As SMRP correlates with bulky disease, a drop in SMRP levels after surgery (EPP) is to be expected (23). The same meta-analysis showed that this marker has also a role in disease progression monitoring, and differentiation between patients with a progressive and stable disease.
The summary of evidence for Fibulin 3 is presented on Table 1 (BTS guidelines). Lower values of Fibulin 3 were shown to be favorable in term of survival (28).
Table 1
| Study | Source | Sensitivity | Specificity | Area under the curve | Cut off (ng/mL) |
|---|---|---|---|---|---|
| Pass et al. (24) 2012. | Plasma | 100 | 100 | 1 | 33 |
| Plasma | 95 | 96 | 0.99 | 53 | |
| Pleural fluid | 84 | 92 | 0.93 | 346 | |
| Agha et al. (25) 2014. | Serum | 88 | 81.8 | 0.776 | 67 |
| Pleural fluid | 72 | 80 | 0.878 | 150 | |
| Elgazzar et al. (26) 2014 | Serum | 100 | 97 | 0.98 | 54 |
| Pleural fluid | 90 | 97 | 0.94 | 520 | |
| Creaney et al. (27) 2014 | Plasma | 22 | 95 | n/a | 52 |
| Plasma | 48 | 71 | 0.671 | 29 | |
| Pleural fluid | 59 | 52 | 0.588 | 346 | |
| Kirschner et al. (28) 2015 | Plasma | 14 | 97 | 29 |
*From reference (19).
Pleural fluid management
Different types of the pleural fluid management in patients with MPM must be analysed in context of the overall therapeutic approach. Having in mind that there is practically no curative treatment for MPM, with controversial role of surgery, the role of palliative treatment, is essential. The PE control is the key part of the palliative treatment for two reasons: first, thoracocenthesis improves or eliminates dyspnea; second, absence of the PE recurrence improves the quality of life (29). In patients unfit for surgery, some kind of pleurodesis should be performed. The prerequisite for any type of pleurodesis is the ability of the lung to expand either by indwelling pleural catheters or chest tube aspiration. The evidence about different types of pleurodesis is given below.
Therapeutic pleural needle-aspiration is usually the first therapeutic procedure. As the fluid re-accumulates, repeated aspirations may be an option, but only in patients with very poor general condition. In other patients, some other procedures are necessary in order to achieve a more efficient control of the PE (30).
Chemical pleurodesis is used the most frequently, usually by applying talc or less frequently, bleomycin or tetracycline (31). Talc can be used either in form of thoracoscopic poudrage or as slurry applied through the chest tube. The success rate is 60–80% in the absence of the trapped lung (32,33).
In a study with a direct comparison of VATS partial pleurectomy (VATS-PP) with talc (poudrage or slurry), despite initial pleurodesis success in favor of VATS-PP group (37% success rate with talc vs. 59% with VATS PP), this trend gradually disappeared during the follow-up period reaching 77% success with talc vs. 70% with VATS PP at one year follow up) (34). Furthermore, VATS pleurectomy brought neither survival benefit nor the lung function improvement. In the VATS group a higher complication rate (31% vs.14%) and longer hospital stay (7 vs. 3 days) were registered.
Concerning the improvement of dyspnea and pleurodesis success, no significant difference between indwelling pleural catheters and talc slurry could be demonstrated in a randomized clinical trial (29).
Concerning the preference between surgical and medical pleurodesis, no clear benefit could be attributed to any of these methods in a retrospective study that included a high number of patients—28.2% vs. 29.7% success rate for medical and surgical group, respectively) (35). The comparison of the success of pleurodesis in patients with MPM with those with PE caused by other malignancies, the success rate was the lowest in patients with MPM—66% vs. 63%, 77% and 74% in patients with lung, breast carcinoma and other malignancies, respectively (36).
Indwelling pleural catheters represent an alternative to pleurodesis. They are equally effective against breathlessness as talc slurry applied through the chest tube, being appropriate for patients with trapped lung as well. If both of the aforementioned options are available, the choice the patient’s preference should be taken into consideration (37).
In brief, talc pleurodesis, performed in form of any of aforementioned ways, seem to be superior to VATS-PP for PE control in patients with mesothelioma.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Malignant Pleural Effusion”. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.02.04/coif). The series “Malignant Pleural Effusion” was commissioned by the editorial office without any funding or sponsorship. DS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of AME Medical Journal from August 2019 to February 2020 and an unpaid Associate Editor-in-Chief of AME Medical Journal from March 2020 to February 2022. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Renshaw AA, Dean BR, Cibas ES, et al. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest 1997;111:106-9. [Crossref] [PubMed]
- Yilmaz U, Polat G, Sahin N, et al. CT in differential diagnosis of benign and malignant pleural disease. Monaldi Arch Chest Dis 2005;63:17-22. [Crossref] [PubMed]
- Wilcox BE, Subramaniam RM, Peller PJ, et al. Utility of integrated computed tomography-positron emission tomography for selection of operable malignant pleural mesothelioma. Clin Lung Cancer 2009;10:244-8. [Crossref] [PubMed]
- Elboga U, Yılmaz M, Uyar M, et al. The role of FDG PET-CT in differential diagnosis of pleural pathologies. Rev Esp Med Nucl Imagen Mol 2012;31:187-91. [Crossref] [PubMed]
- Porcel JM, Hernández P, Martínez-Alonso M, et al. Accuracy of fluorodeoxyglucose-PET imaging for differentiating benign from malignant pleural effusions: a metaanalysis. Chest 2015;147:502-12. [Crossref] [PubMed]
- Bénard F, Sterman D, Smith RJ, et al. Metabolic imaging of malignant pleural mesothelioma with fluorodeoxyglucose positron emission tomography. Chest 1998;114:713-22. [Crossref] [PubMed]
- Abe Y, Tamura K, Sakata I, et al. Clinical implications of 18F-fluorodeoxyglucose positron emission tomography/computed tomography at delayed phase for diagnosis and prognosis of malignant pleural mesothelioma. Oncol Rep 2012;27:333-8. [PubMed]
- Yildirim H, Metintas M, Entok E, et al. Clinical value of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiation of malignant mesothelioma from asbestos-related benign pleural disease: an observational pilot study. J Thorac Oncol 2009;4:1480-4. [Crossref] [PubMed]
- Plathow C, Staab A, Schmaehl A, et al. Computed tomography, positron emission tomography, positron emission tomography/computed tomography, and magnetic resonance imaging for staging of limited pleural mesothelioma: initial results. Invest Radiol 2008;43:737-44. [Crossref] [PubMed]
- Revelli M, Chiesa F, Del Prato A, et al. Role of respiratory-triggered diffusion-weighted MRI in the assessment of pleural disease. Br J Radiol 2016;89:20160289. [Crossref] [PubMed]
- Coolen J, De Keyzer F, Nafteux P, et al. Malignant pleural mesothelioma: visual assessment by using pleural pointillism at diffusion-weighted MR imaging. Radiology 2015;274:576-84. [Crossref] [PubMed]
- Gill RR, Umeoka S, Mamata H, et al. Diffusion-weighted MRI of malignant pleural mesothelioma: preliminary assessment of apparent diffusion coefficient in histologic subtypes. AJR Am J Roentgenol 2010;195:W125-30. [Crossref] [PubMed]
- Husain AN, Colby TV, Ordonez NG, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma: 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch Pathol Lab Med 2018;142:89-108. [Crossref] [PubMed]
- Hwang HC, Sheffield BS, Rodriguez S, et al. Utility of BAP1 Immunohistochemistry and p16 (CDKN2A) FISH in the Diagnosis of Malignant Mesothelioma in Effusion Cytology Specimens. Am J Surg Pathol 2016;40:120-6. [Crossref] [PubMed]
- Hiroshima K, Wu D, Hasegawa M, et al. Cytologic Differential Diagnosis of Malignant Mesothelioma and Reactive Mesothelial Cells With FISH Analysis of p16. Diagn Cytopathol 2016;44:591-8. [Crossref] [PubMed]
- Berg KB, Churg A. GATA3 Immunohistochemistry for Distinguishing Sarcomatoid and Desmoplastic Mesothelioma From Sarcomatoid Carcinoma of the Lung. Am J Surg Pathol 2017;41:1221-5. [Crossref] [PubMed]
- Churg A, Sheffield BS, Galateau-Salle F. New Markers for Separating Benign From Malignant Mesothelial Proliferations: Are We There Yet? Arch Pathol Lab Med 2016;140:318-21. [Crossref] [PubMed]
- Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 2015;28:1043-57. [Crossref] [PubMed]
- Woolhouse I, Bishop L, Darlison L, et al. BTS guidelines for the investigation and management of malignant pleural mesothelioma. Thorax 2018;73:i1-30. [Crossref] [PubMed]
- Creaney J, Yeoman D, Demelker Y, et al. Comparison of osteopontin, megakaryocyte potentiating factor, and mesothelin proteins as markers in the serum of patients with malignant mesothelioma. J Thorac Oncol 2008;3:851-7. [Crossref] [PubMed]
- Creaney J, Robinson BW. Detection of malignant mesothelioma in asbestos-exposed individuals: the potential role of soluble mesothelin-related protein. Hematol Oncol Clin North Am 2005;19:1025-40. [Crossref] [PubMed]
- Cui A, Jin XG, Zhai K, et al. Diagnostic values of soluble mesothelin-related peptides for malignant pleural mesothelioma: updated meta-analysis. BMJ Open 2014;4:e004145. [Crossref] [PubMed]
- Creaney J, Francis RJ, Dick IM, et al. Serum soluble mesothelin concentrations in malignant pleural mesothelioma: relationship to tumor volume, clinical stage and changes in tumor burden. Clin Cancer Res 2011;17:1181-9. [Crossref] [PubMed]
- Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 2012;367:1417-27. [Crossref] [PubMed]
- Agha MA, El-Habashy MM, El-Shazly RA. Role of fibulin-3 in the diagnosis of malignant mesothelioma. Egypt J Chest Dis Tuberc 2014;63:99-105. [Crossref]
- Elgazzar Aeldin M, Embarak S, Refat AM, et al. Value of plasma and pleural effusion fibulin-3 levels in the diagnosis of malignant pleural mesothelioma effusions. Egypt J Chest Dis Tuberc 2014;63:883-8. [Crossref]
- Creaney J, Dick IM, Meniawy TM, et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 2014;69:895-902. [Crossref] [PubMed]
- Kirschner MB, Pulford E, Hoda MA, et al. Fibulin-3 levels in malignant pleural mesothelioma are associated with prognosis but not diagnosis. Br J Cancer 2015;113:963-9. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Bibby AC, Gibbs L, Braybrooke JP. Medical and oncological management of malignant mesothelioma. Br J Hosp Med (Lond) 2015;76:384-9. [Crossref] [PubMed]
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001;18:402-19. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii32-40. [Crossref] [PubMed]
- Kennedy L, Rusch VW, Strange C, et al. Pleurodesis using talc slurry. Chest 1994;106:342-6. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Fysh ET, Tan SK, Read CA, et al. Pleurodesis outcome in malignant pleural mesothelioma. Thorax 2013;68:594-6. [Crossref] [PubMed]
- Bielsa S, Hernández P, Rodriguez-Panadero F, et al. Tumor type influences the effectiveness of pleurodesis in malignant effusions. Lung 2011;189:151-5. [Crossref] [PubMed]
- Maskell NA. Treatment options for malignant pleural effusions: patient preference does matter. JAMA 2012;307:2432-3. [Crossref] [PubMed]
Cite this article as: Subotic D. Malignant pleural effusion and mesothelioma. AME Med J 2020;5:11.

