
Bladder preservation therapy in muscle-invasive bladder cancer: Current evidence and future perspectives
Introduction
Bladder cancer is the ninth most common malignancy worldwide (1). About 430,000 new bladder cancer cases were diagnosed in 2012, and a substantial increase of this incidence was noted in the elderly adults (2). Muscle invasive bladder cancer (MIBC), which accounts for approximately 25–30% of all bladder cancer diagnoses, requires more aggressive management as compared to non-muscle invasive cancer (2), and radical cystectomy (RC) has been widely accepted as the standard treatment for cT2-4aN0M0 bladder cancer (3). The current guidelines also recommend RC for patients with high-risk non-muscle invasive bladder cancer with a strong rating (3). However, removal of the bladder requires urinary diversions that can substantially impair patients’ quality of life (QOL) post surgery. Concerns regarding urinary diversions also include possible postoperative impairment of renal function as previously reported in several studies (4,5). Furthermore, RC is associated with a significant risk of perioperative complications and mortality, which makes certain MIBC patients unfit for RC (6). In a previous study which analyzed the data from the National Cancer Data Base in the United States, overall 30- and 90-day postoperative mortality after RC was 2.7% and 7.2%, respectively (7). The authors interestingly pointed out that a low hospital volume was associated with greater 30- and 90-day mortality. This dilemma is more apparent in elderly patients, where perioperative mortality significant increases as compared to younger patients (8,9). Despite the higher incidence of bladder cancer in the elderly population, previous studies revealed that only a minority (20–30%) of MIBC patients aged 70 years or older actually received RC in real world (10,11). Given the recently extended life expectancy, the demand for alternative curative treatment for elderly MIBC patients who are unfit for RC is increasing.
Bladder preservation therapy (BPT) for MIBC patients has been explored as a potential alternative to overcome such limitations of RC. A growing body of evidence suggests that BPT could become one of the major therapeutic interventions in a sub-set of MIBC patients. This review provides an overview of the current practices, efficacy, limitations, and future perspectives on BPT.
Development of chemoradiation-based bladder preservation therapy
Earlier, bladder preservation approaches for MIBC included transurethral resection of the bladder tumor (TURBT), radiation therapy, or chemotherapy alone. However, such single-modality treatments failed to achieve the desired clinical outcomes (12-15). Subsequently, several randomized trials demonstrated the advantages of radiotherapy with concurrent chemotherapy (chemoradiotherapy, CRT) over radiotherapy alone in achieving local cancer control (16,17). In the late 1980s, pioneering medical centers developed a combinatorial treatment plan for MIBC patients that included maximal TURBT followed by radiation therapy with concurrent chemotherapy (trimodality therapy: TMT). TMT achieved a complete response in 64–74% of patients (18-20). One of the key features of the TMT was an optimal patient selection based on induction or full dose of CRT response, whereby the bladder conservation was reserved only for complete CRT responders.
To date, the TMT has been well investigated in several prospective studies, demonstrating five-year overall survival rates to be 50–70%, which is comparable to five-year overall survival rates in patients treated with RC (17,19,21-24) (Table 1). These studies also showed that 40–60% of patients survived with preserved bladder after undergoing BPT. A growing body of evidence demonstrated benefits and efficacy of CRT-based bladder preservation protocols, leading to recognition of the BPT, according to the 2017 US guideline (AUA/ASCO/ASTRO/SUO Guideline), as one of the standard options for MIBC patients (25). The current NCCN guideline also proposes CRT as a viable option for cystectomy candidates as well as neoadjuvant chemotherapy followed by RC (26), suggesting that the BPT is now being acknowledged as one of the primary treatment strategies in MIBC patients. In contrast, the EAU guideline emphasizes the importance of patient selection for bladder preservation to achieve a favorable long-term survival outcomes, although it allows multimodality BPT to be performed as a primary therapeutic intervention (3). The EAU guideline also highlighted the importance of a close multidisciplinary cooperation between urologists, oncologists, and radiotherapists, and pointed out the need of a high level of patient compliance and wide patient counseling to appropriately manage the potential risk of recurrence in preserved bladders (3).
Table 1
| Investigator (publication year) | # of patients | Clinical stage | CRT regimen | Protocol types: split or continuous | CR rate (%) | 5-year bladder preservation rate (%) | 5-year OS rate (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| James (2012) | 182 | T2-4a | 55 Gy or 64 Gy + 5-FU, MMC | Continuous | – | 88 (2-year) | 48 | (17) |
| Housset (1993) | 54 | T2-4 | 44 Gy + cisplatin, 5-FU | Split | 74 | – | 59 (3-year) | (19) |
| Shipley (2002) | 190 | T2-4a | 64.8 Gy + cisplatin | Split | 64 | 46 | 54 | (21) |
| Rödel (2002) | 289 | T1-4 | 50.4 Gy + cisplatin or carboplatin | Continuous | 72 | 42 | 50 | (22) |
| Tunio (2012) | 200 | T2-4 | 65 Gy + cisplatin | Continuous | 93 | – | 52 | (23) |
| Weiss (2007) | 112 | T1-4 | 55.8-59.4 Gy + cisplatin, 5-FU | Continuous | 88 | 61 | 74 | (24) |
5-FU, 5-fluorouracil; CR, complete response; CRT, chemoradiotherapy; CSS, cancer-specific survival; MMC, mitomycin C; OS, overall survival.
Current practices in chemoradiation-based bladder preservation therapy
Optimal candidates
BPT used to be an alternative for medically inoperable MIBC patients who are unfit for RC due to severe comorbidities and/or poor physical conditions. However, BPT is currently opened also for operable MIBC patients based on their preferences. As optimal patient selection is essential to avoid the impairment of patient survival by an imprudent de-escalation of definitive treatment, the current TMT protocols generally allow bladder preservation only in patients who achieved a complete response to CRT. Furthermore, previous studies showed significantly worse survival outcomes in CRT non-responders as compared to CRT responders (27-30), suggesting that ideal candidates for bladder preservation are patients whose bladder cancers could be completely controlled by CRT.
The clinical tumor parameters associated with unfavorable CRT response include large tumor size (>5 cm), locally advanced stage (≥ T3), multifocal disease, and the presence of hydronephrosis (22,31,32). Previous studies suggested that tumor multiplicity and concomitant carcinoma in situ (CIS) are recurrence risk factors post BPT (22,31,32). Hydronephrosis has been also identified as a prognostic factor associated with a poor CRT response, and several studies have recommended hydronephrosis as an exclusion criterion for BPT (21,30). All of the evidence highlighted that a limited number of MIBC patients meet the ideal criteria for bladder preservation. A previous review demonstrated that 10–15% of medically operable patients were good candidates for BPT (30).
While the utility of CRT is widely acknowledged in muscle-invasive urothelial carcinoma, the impacts of histologic variants on treatment outcomes remains elusive. Krasnow et al. recently analyzed 303 bladder cancer patients treated with the TMT and showed that 66 (22%) patients had histologic variants (squamous and/or glandular differentiation in 50 patients and other forms in 16) (33). These data suggest that histological variants have no bearing in CRT response, salvage cystectomy, and survival rates. Thus, the current practice of CRT-based bladder preservation allows MIBC patients with histologic variants to be included as well as those with pure urothelial carcinoma. Further studies are required to elucidate if different treatment plans should be enacted for MIBC patients with histologic variants.
Therapeutic protocols
Split versus continuous protocols
TMT comprising maximal TURBT followed by CRT is the current mainstream of the CRT-based bladder preservation approach. The current TMT protocols included two types of therapeutic courses: split or continuous course (Figure 1). A common practice between the two types of protocols is to offer prompt salvage RC for patients whose tumors do not respond to CRT. CRT response is generally assessed on cystoscopy, urine cytology, radiological examination, and/or transurethral biopsy of the bladder mucosa.
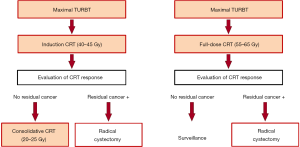
In the split protocol, treatment response is evaluated after induction CRT with 40–45 Gy radiation, and only patients who achieve complete response undergo consolidative CRT with 20–25 Gy radiation; otherwise, salvage RC is recommended (30,34). This strategy enables an early patient selection based on the CRT sensitivity and thus may improve overall outcomes by minimizing the delay of salvage RC. In contrast, the continuous protocol consists of full-dose CRT with 55–65 Gy radiation followed by response evaluation, which may maximize the chance of bladder preservation because all candidates undergo full-dose CRT (30,34). The caveats of this approach are a potential negative impact of delayed RC and an increased risk of RC-related complications after the full-dose pelvic irradiation (35).
Radiation regimens
Although most of the planned CRT regimens for bladder preservation include radiation to a limited pelvic field (typically around the mid-sacroiliac region with an upper limit at the common iliac artery bifurcation), the survival benefits of including pelvic nodal packets remains unclear (23). In the current regimens, a total dose of 64–65 Gy is directed to the whole bladder and tumor, while the nodal packets are subjected to a lower radiation dose (40–45 Gy) to potentially conserve the small bowel for future urinary diversions, if necessary (30). Currently, viable regimens include once or twice daily radiation fractions, though the difference of fractionation has not been proved to be a prognostic factor (36). The Radiation Therapy Oncology Group (RTOG) 0712 study, a randomized phase 2 trial, compared twice-a-day radiation with fluorouracil/cisplatin and once-a-day radiation with gemcitabine, and showed that favorable outcomes were observed in both groups, suggesting that either regimen could serve as a base for future trials (37).
Concurrent chemotherapy
Cisplatin has been well studied as an active single agent that may improve local cancer treatment in combination with radiotherapy (16,21-23). To date, concurrent administration of cisplatin as a radiosensitizing drug has been the standard care in most CRT-based bladder preservation protocols for patients with adequate renal function, although a phase 3 trial comparing cisplatin versus non-cisplatin regimens has not been performed (30). Previous studies failed to show significant associations between different schedules or concentrations of cisplatin administration with treatment outcomes (16,21-23,36). As a potential alternative for cisplatin-unfit patients, a previous randomized phase 3 trial demonstrated that the combination of fluorouracil and mitomycin C improved regional cancer control when compared to radiation alone (17). Low-dose gemcitabine has also been studied as a radiosensitizer for the TMT in phase 1-2 trials (38,39). In the most recent randomized phase 2 trial (RTOG 0712), once-daily radiation plus gemcitabine was shown to yield favorable outcomes and safety profiles in comparison to cisplatin-based CRT regimen (37).
Efficacy and limitations
Oncologic outcomes
In Table 1, we reviewed previous studies that include a substantial number of patients (n≥50) who were treated with the specific regimen of TMT. The data showed five-year overall survival rates to be 50–70%. Additionally, 40–60% of patients survived with preserved bladder post treatment. The Massachusetts General Hospital team, one of the pioneering centers in developing the TMT protocol, previously reported long-term outcomes of BPT in 348 patients, who were treated with several kinds of CRT-based regimen at a single institution (36). With a median follow-up of 7.7 years, they demonstrated that the complete response, bladder preservation and five-year overall survival rates were 72%, 71%, and 52%, respectively. The five-year overall survival rates for CRT responders and non-responders were approximately 60% and 40%, respectively, indicating that the CRT response is a strong predictor of patient survival and should be used to select MIBC patients for BPT.
Although previous studies demonstrated comparable survival outcomes in CRT-based BPT and RC (40,41), the impacts of bladder preserving option over RC on patient survivals remains elusive due to lack of randomized control trials. Recently, two retrospective studies used propensity score to compare CRT and RC in MIBC patients (National Cancer Database), and showed there were insufficient survival outcomes in patients treated with CRT (42,43). Interestingly, Kulkarni et al. performed propensity score-matched analysis in MIBC patients who underwent either TMT or RC, based on the judicious assessment by a multidisciplinary team consisting of bladder cancer experts (44), and showed that there was no significant difference in the survival rates of the TMT and RC groups. With the lack of randomized control trials, propensity score-matched analysis used in these studies may reduce the impact of inevitable selection biases between the BPT and RC groups by creating samples of units that are comparable on all covariates to each other.
Quality of life after treatment
There have been two prospective trials that confirmed satisfactory QOL and bladder function after BPT, although the numbers of patients were limited in the both studies (45,46). Zietman et al. evaluated bladder function after TMT by the cross-sectional analysis using urodynamic study, and demonstrated that 75% of patients had normally functioning bladder after treatment, while decreased bladder compliance, bladder hypersensitivity, involuntary detrusor contractions or incontinence were observed in the minority (47). A retrospective study by Henningsohn et al. highlighted sexual function after radiotherapy for MIBC. They reported that 38% of patients had intercourse in the previous month and 57% of male patients had preserved ejaculation (48). Caffo et al. retrospectively compared the results of QOL questionnaires between the patient groups undergoing BPT and RC, which revealed that QOL after RC was reduced particularly by lacking sexual activity and worsened physical conditions in comparison to BPT, while the items regarding social and recreational life were little affected by the types of treatment (49).
Limitations
Limitations related to the current practice of CRT-based bladder preservation protocols include a significant risk of MIBC recurrence in the preserved bladders, which would demand salvage RC. The MIBC recurrence rate was reported to be 11–19% in previous studies, even though a complete remission of cancer was clinically confirmed following CRT in all patients (17,19,21-24,30,36). Since most of the MIBC recurrences develop at the original MIBC site, it is possible that small foci of bladder cancer remnants could have evaded diagnosis post CRT. These foci could subsequently grow in size and result in MIBC recurrence. Another potential limitation of BPT is the delay of RC, which might negatively impact patients’ prognosis, as demonstrated by significantly worse survival outcomes in CRT non-responders as compared to CRT responders (27-30). Furthermore, salvage RC after BPT generally precludes orthotopic neobladder reconstruction as urinary diversion (30). Importantly, the optimal indication for the current CRT-based BPT and RC with orthotopic neobladder reconstruction is largely overlapped (e.g., the absence of bladder neck involvement or CIS), and the accurate prediction of CRT sensitivity before the treatment induction may provide MIBC patients with CRT resistance with the opportunity to select RC with orthotopic neobladder reconstruction instead of BPT. Different treatment approaches should be offered to patients whose tumors are potentially resistant to CRT since clinicians’ ability to predict CRT response remains limited even when potential risk factors are identified. (22,31,32). As such, further studies are required to establish personalized treatment plans in some MIBC patients.
Molecular biomarkers in bladder preservation therapy
Clinicopathological factors play an important role in predicting better therapeutic outcomes post CRT-based BPT. However, these clinicopathological factors are insufficient to predict CRT response and outcomes after bladder preservation. Therefore, molecular biomarkers that can predict precise therapeutic outcomes are strongly warranted. Recent understanding of the molecular and genomic characteristics of bladder cancer has led to the identification of different molecular biomarkers of MIBC. In this chapter, we will discuss the possible prognostic or predictive biomarkers of BPT in MIBC patients.
DNA repair gene alteration
MIBCs are characterized by a complex genomic landscape, such as high mutation burden, frequent copy number alteration, and chromosomal translocations (50). These genomic instabilities are caused in part by alterations in the DNA repair pathway, which could also affect patients’ sensitivity to CRT-based treatments (51). Therefore, factors or genes involved in the DNA repair pathway have been investigated as potential biomarkers to predict outcomes in MIBC patients.
Ionizing radiation causes DNA double-strand breaks and consequently induces cell death. Double-strand break is detected by meiotic recombination 11 (MRE11)-RAD50-NBS1 complex which activate the DNA double-strand break repair pathway (52). Additionally, platinum agents produce DNA intrastrand adducts, which could be repaired by the nucleotide excision repair pathway, a highly conserved DNA repair mechanism. Excision repair cross-complementing group 1 (ERCC1) is one of the key players in the nucleotide excision repair pathway (53).
MRE11
MRE11 is one of the most investigated DNA repair biomarkers in MIBC. In response to DNA double-strand breaks, MRE11 interact with its binding partners RAD50 and NBS1 to coordinate DNA repair at damage sites and maintain DNA integrity (53). Choudhury et al. investigated MRE11 expression immunohistochemically in MIBC patients treated with definitive radiotherapy, and reported that a higher MRE11 expression was associated with better survival outcomes (hazard ratio 0.36, P=0.01) (54). Although no significant association was found between MRE11 expression and survival outcomes in cystectomy cohorts, patients with higher MRE11 expression showed better survival outcomes in patients treated with radiotherapy than cystectomy (hazard ratio 0.60, P=0.02). These data suggest that MRE11 expression might be predictive of radiotherapy response.
A similar association between high MRE11 expression and better outcomes has also been reported in invasive bladder cancer patients receiving CRT-based BPT. Laurberg et al. reported that high MRE11 expression was associated with better survival outcomes in 148 patients treated with radiotherapy (17%) or CRT (83%); however, there was no significant association between MRE11 expression and survival outcomes in 273 patients treated with cystectomy (55). Magliocco et al. evaluated associations between MRE11 expression and outcomes in 135 patients from 6 NRG/RTOG TMT trials, and revealed that patients with MRE11 nuclear-to-cytoplasm rate in the lowest quartile (≤1.49) had significant higher cancer-specific mortality (56). These data consistently suggested that MRE11 could be a potential biomarker for selection of patients most likely to respond to CRT-based BPT. A recent study by Walker et al. highlighted an important caveat of the role of MRE11 as a biomarker for CRT response (57). In this study, the authors failed to validate MRE11 expression assessed on immunohistochemistry as a prognostic marker in patients managed with CRT because of the lack of a reproducible assay for MRE11 immunohistochemistry. They demonstrated the challenges involved in developing a robust immunohistochemistry-based protein biomarker.
Nucleotide excision repair pathway genes (ERCC1 and ERCC2)
The association between expression of nucleotide excision repair pathway genes and MIBC outcomes has been primarily investigated in patients treated with chemotherapy as compared to patients treated with radiotherapy. Bellmunt et al. reported that low level of ERCC1 mRNA was associated with improved survival outcomes in metastatic bladder cancer patients treated with platinum-based chemotherapy, suggesting that a loss in the nucleotide excision capacity improves platinum sensitivity (58). In contrast, Sakano et al. reported that high expression of ERCC1 and X-ray repair cross-complementing group 1 (XRCC1), which is a key protein in DNA single-strand break repair pathway, was associated with improved survival outcomes (59). Therefore, the effect of ERCC1 on treatment outcome may vary across clinical settings and further studies are warranted to define the role of ERCC1 as a biomarker for CRT-based BPT.
The development of next-generation sequencing has identified structural alterations in the DNA repair pathway genes in bladder cancer patients, and showed that these alternations could be associated with sensitivity to cisplatin-based chemotherapy (60). Desai et al. evaluated the association between such alterations and survival outcomes in 48 patients who underwent CRT-based BPT, and showed that patients with deleterious mutations in one or more DNA repair genes were associated with reduced recurrence rate, and in particular, those with ERCC2 mutations had lower rate of metastasis (hazard ratio 0.47, P=0.04) (61). These results suggested that structural alterations in genes involved in the DNA repair pathway could be used as potential biomarkers to predict favorable outcomes in patients treated with CRT-based BPT.
Signal transduction pathway
In addition to DNA repair pathways, signal transduction pathways are frequently altered in bladder cancer, and could serve as potential biomarkers for CRT response.
EGFR
The receptor tyrosine kinase EGFR is altered in more than 30% of bladder cancer patients (62). Chakravarti et al. reported that EGFR expression was positively associated with survival outcomes (hazard ratio 0.12, P=0.04) in a cohort of 73 patients enrolled in 4 prospective CRT-based BPT with cisplatin (63).
HER2
In contrast, HER2 expression was negatively associated with complete response in the same cohort of patients used in Chakravarti et al. (50% vs. 81%, P=0.03) (63). In line with this result, Inoue et al. reported that HER2 overexpression was a significant predictor of incomplete response (odds ratio 2.9, P=0.03) and shorter five-year survival rates (56% vs. 87%, P=0.001) (64).
VEGF
Angiogenesis plays a crucial role in bladder cancer development, progression, and metastasis. Elevated levels of VEGF, a key signal transducer of angiogenesis, have been associated with increased recurrence and metastasis rates in bladder cancer (65). Lautenschlaeger et al. evaluated the association between VEGF expression and survival outcomes in 43 patients from 4 prospective bladder preservation studies and showed that VEGF expression was not associated with complete response to CRT. However, overexpression of VEGF-B, VEGF-C, and their receptor VEGF-R2 was negatively associated with survival outcomes (66). Keck et al. showed that overexpression of both VEGF-C and its receptor neuropilin-2 (NRP2) was negatively associated with survival outcomes in a cohort of 247 patients treated with CRT-based BPT (67). Collectively, these results suggest that hyperactivation of VEGF signaling is associated with poor survival outcomes post BPT.
Cellular proliferation markers
Cellular proliferation markers have been investigated as potential BPT biomarkers. Ki-67 is an established marker of cellular proliferation (68). Rödel et al. reported that Ki-67 expression positively correlates with complete response rate and survival outcomes in 70 patients receiving CRT-based BPT (69). Similarly, Tanabe et al. reported that high Ki-67 expression was associated with improved survival outcomes (five-year survival rate 78% vs. 46%, P=0.02) in 94 patients receiving CRT-based BPT (70). The apparent diffusion coefficient (ADC) is a potential radiological marker that reflects cellular proliferation, and is measured by diffusion-weighted magnetic resonance imaging (71). Yoshida et al. reported that ADC values are inversely correlated with Ki-67 expressions, and lower ADC values predicted a better response to CRT-based BPT (72). In contrast, Matsumoto et al. previously reported conflicting results regarding Ki-67 expression and CRT response (73). Further studies were required to validate the prognostic impact of Ki-67 expression on CRT sensitivity of bladder cancer.
Hypoxia
Since oxygen plays an important role in the induction of DNA damage resulting from ionizing radiation, hypoxia or reduced oxygen level is believed to result in resistance to radiotherapy (74). For instance, hypoxia modification with carbogen and nicotinamide (CON) improved prognostic outcomes in invasive bladder cancer patients treated with radiotherapy (75). In addition, Yang et al. reported that a 24-gene hypoxia signature could be used for predicting prognostic outcomes in MIBC patients receiving radiotherapy with CON (76). Thus, this gene signature could be a biomarker to identify a subset of patients who would most benefit from hypoxia modification.
Molecular subtypes
Recent genome-wide gene-expression studies of bladder cancer patients have identified distinct molecular subtypes (62). These molecular subtypes can predict outcomes for neoadjuvant cisplatin-based chemotherapy. For instance, basal type bladder cancer is associated with shorter survival after cystectomy, but has substantial survival improvement with chemotherapy (77). Unfortunately, the predictive role of molecular subtypes in CRT-based BPT has not been well studied.
In a transcriptome wide gene expression analysis in 103 patients treated with CRT-based BPT, molecular subtypes were not significantly associated with survival outcomes. However, several gene signatures correlated with CRT response, suggesting that genome-wide analyses could play a role in identifying optimal MIBC patients for TMT (78). Tanaka et al. evaluated the predictive utility of immunohistochemistry-based molecular subtypes in CRT-based BPT, and reported that genomically unstable (GU) and squamous cell cancer-like (SCCL) subtypes showed more favorable CRT response than urobasal (Uro) subtypes (79,80).
All these prognostic or predictive biomarkers of BPT in MIBC patients described above are summarized in Table 2.
Table 2
| Biomarkers | Methods | Patients | Findings | Reference |
|---|---|---|---|---|
| DNA repair gene alterations | ||||
| MRE11 | Immunohistochemistry | 181 in RT, 88 in RC | RT: High MRE11 associated with better CSS | (54) |
| RC: MRE11 not associated with CSS | ||||
| MRE11 | Immunohistochemistry | 148 in CRT, 273 in RC | CRT: High MRE11 associated with better CSS | (55) |
| RC: MRE11 not associated with CSS | ||||
| MRE11 | Immunohistochemistry (nuclear to cytoplasm ratio) | 135 in CRT | CRT: Low MRE11 nuclear/cytoplasmic ratio associated with higher mortality | (56) |
| ERCC1 | Immunohistochemistry | 157 in CRT | CRT: High ERCC1 and XRCC1 associated with better CSS | (59) |
| ERCC2 | Next-generation sequencing | 48 in CRT | CRT: ERCC2 mutation associated with less metastases | (61) |
| Signal transduction pathways | ||||
| EGFR | Immunohistochemistry | 73 in CRT | CRT: High EGFR associated with improved CSS | (63) |
| HER2 | Immunohistochemistry | 119 in CRT | CRT: High HER2 associated with shorter CSS | (64) |
| VEGF-B, VEGF-C, VEGF-R2 | Immunohistochemistry | 43 in CRT | CRT: High VEGF-B, VEGF-C, and VEGF-R2 associated with lower OS | (66) |
| VEGF-C, NRP2 | Immunohistochemistry | 247 in CRT | CRT: High VEGF-C and NRP2 associated with lower CSS | (67) |
| Cellular proliferation marker | ||||
| Ki-67 | Immunohistochemistry | 70 in CRT | CRT: High Ki-67 associated with better CR rate and CSS | (69) |
| Ki-67 | Immunohistochemistry | 94 in CRT | CRT: High Ki-67 associated with better CSS | (70) |
| ADC | Diffusion weighted MRI | 23 in CRT | CRT: Low ADC associated with high Ki-67 and better sensitivity to CRT | (72) |
| Hypoxia | ||||
| Hypoxia gene signature | RNA expression microarray | 75 in RT 76 in RT+CON |
RT: Hypoxia signature associated with lower relapse-free survival | (76) |
| RT + CON: Hypoxia signature associated benefit from addition of CON to RT | ||||
| Molecular subtypes | ||||
| Molecular subtypes | Immunohistochemistry | 118 in CRT | CRT: GU and SCCL subtypes had better CRT response than Uro subtype | (80) |
CON, carbogen and nicotinamide; CRT, chemoradiotherapy; CSS, cancer-specific survival; GU, genomically unstable; OS, overall survival; RC, radical cystectomy; RT, radiotherapy; SCCL, squamous cell cancer-like; Uro, urobasal.
Future perspectives on bladder preservation therapy
Neoadjuvant or adjuvant chemotherapy along with trimodality therapy
While the positive impact of neoadjuvant chemotherapy prior to RC on oncologic outcomes in MIBC patients is widely acknowledged (81), there has been no definitive evidence regarding its utility in BPT. In the single institutional large series (n=348), the Massachusetts General Hospital team analyzed MIBC patients who underwent TMT with or without neoadjuvant chemotherapy (methotrexate, cisplatin, and vinblastine), and showed that there was no significant association between the use of neoadjuvant chemotherapy and survival outcomes (36). In contrast, a separate study in 104 MIBC patients, who underwent neoadjuvant therapy with gemcitabine and cisplatin followed by CRT (60–65 Gy), showed favorable local cancer control and survival outcomes with complete response, five-year cancer-specific survival and five-year overall survival rates of 79%, 76%, and 68%, respectively (82). Most recently, Jiang et al. retrospectively analyzed 57 MIBC patients treated neoadjuvant chemotherapy (gemcitabine and cisplatin) followed by CRT (60–66 Gy), and demonstrated encouraging outcomes and tolerability with overall survival rate of 74% and disease-specific survival of 88% at two years (83). Several phase 1-2 trials in MIBC patients receiving adjuvant chemotherapy post CRT demonstrated five-year overall survival rate to be 56–73% (84,85). Further studies are warranted to define the potential role of neoadjuvant or adjuvant chemotherapy in BPT.
Combination of consolidative surgery and CRT (tetramodality bladder preservation therapy)
CRT response is generally assessed on cystoscopy, urine cytology, radiological examination, and/or transurethral biopsy of the bladder mucosa (clinical CRT response). However, such clinical evaluations could miss small foci of cancer remnants at the original MIBC site, which may lead to MIBC recurrence after TMT (30). In 1997, we developed a tetramodality bladder preservation protocol comprising of maximal TURBT, induction CRT, and consolidative partial cystectomy of the original MIBC site with pelvic lymph node dissection, which allows for pathological confirmation of CRT response (pathological CRT response) (27) (Figure 2).
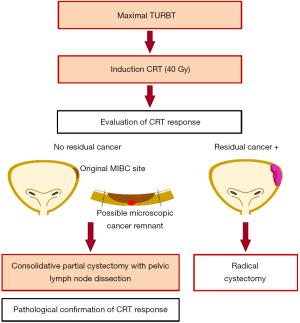
Our tetramodality protocol includes induction CRT (40 Gy radiation with concurrent cisplatin administration) followed by consolidative partial cystectomy and pelvic lymph node dissection when complete remission of MIBC is clinically confirmed. Our protocol proposes salvage RC instead, when complete remission is not achieved. The clinical evaluation of CRT response consists of bladder magnetic resonance imaging, urine cytology, cystoscopy, and restaging transurethral resection performed 4–6 weeks post CRT completion. The inclusion criteria are (I) pathologically confirmed urothelial carcinoma, (II) circumscription within ≤25% of the bladder surface, (III) absence of bladder neck involvement, and (IV) absence of broad CIS. Recently, we reported oncological and functional outcomes in 154 patients whose tumor met all the criteria described above, which accounted for 49% of all MIBC patients (n=312) treated at our institution during the same period (86). Most frequent reason for exclusion was bladder neck involvement (n=73) followed by the presence of broad CIS (n=36). A clinical complete response was observed in 121 patients (79%) of the 154 patients post CRT, and 107 patients (69%) underwent consolidative partial cystectomy and achieved bladder preservation according to the tetramodal protocol. Pathological examinations in these 107 patients revealed residual invasive cancer remnants (≥ pT1) in 11 patients (10%) and lymph node metastases in 2 patients (2%). These cancer remnants were surgically removed and we believe that the consolidative partial cystectomy could have contributed to preventing MIBC recurrence in these patients. The overall MIBC recurrence rate, noted over a median follow-up period of 48 months, in 107 patients was low (4%). Intention-to-treat analyses in all of the 154 enrolled patients demonstrated favorable survival outcomes (5-year cancer-specific and overall survival rates: 83% and 77%, respectively). Functional analyses revealed that patients treated with tetramodality therapy maintained high QOL and preserved bladder functions (86). We further evaluated the annual change of renal function of patients who underwent tetramodality therapy, demonstrating that post-treatment renal function was well preserved over time (87). It should be noted that partial cystectomy on MIBC patients is generally not recommended by the current guidelines. Further follow up is warranted to confirm the favorable oncologic outcomes after this procedure. Although, our results suggest that consolidative partial cystectomy, along with pelvic lymph node dissection, could be a useful adjunct in BPT, further studies are required to establish the consensus about this surgical option.
Combination of immunotherapy and bladder preservation therapy
The combination of immune checkpoint inhibitors with CRT-based BPT could be a promising approach in achieving a synergistic anti-tumor activity between radiation and immunotherapy (88). Evidence from other types of malignancies support such a combinatorial strategy. For instance, the PACIFIC trial, which evaluated patients with non-small-cell lung cancer treated with definitive CRT and subsequently randomized to receive checkpoint inhibitors durvalumab or placebo, reported significant improvement in survival outcomes in the durvalumab group (89).
Tumor DNA repair deficiency has been studied as a potential biomarker for immune checkpoint inhibitors (90), and among them, the most well established biomarker is the mismatch repair deficiency (91). Although the association between other DNA repair pathways and efficacy of immune checkpoint inhibitors are less established, several recent studies reported an association between alteration in DNA repair genes and improved response to immune checkpoint inhibitors (92). These data suggest that combinatorial use of immune checkpoint inhibitors and CRT in treating patients with bladder cancer that resulted from alterations in DNA repair pathways, such as the mismatch, the DNA double-strand break, and the nucleotide excision repair pathways, could yield beneficial results.
There are several ongoing clinical trials of BPT comprising immune checkpoint inhibitors (Table 3). NCT02621151 and NCT02622262 are single-arm phase II trials evaluating the combination of pembrolizumab with CRT using gemcitabine (NCT02621151) or cisplatin (NCT02662062). NCT02891161 is a phase 1-2 study evaluating the safety and efficacy of the combination of durvalumab and radiation therapy. NCT03993249 is a randomized phase II study comparing the combination of standard CRT and nivolumab. Additionally, SWOG trial (NCT03775265)—a randomized phase III study—plans to enroll 475 patients and evaluate CRT-based BPT with and without atezolizumab. The treatment regimen will include radiation therapy, chemotherapy based on physician's choice and anti-PD-L1 monoclonal antibody atezolizumab (or placebo). This study will give us more definitive evidence whether the addition of immunotherapy to CRT will increase the chance of successful bladder preservation in MIBC patients.
Table 3
| Trial | Phase | Checkpoint inhibitors | Chemotherapy | N | Arm | Primary endpoint | Secondary endpoint |
|---|---|---|---|---|---|---|---|
| NCT02621151 | 2 | Pembrolizumab | Gemcitabine | 54 | Pembro + Gem + EBRT | 2-years BIDFS | AE, CR, OS, MFS |
| NCT02662062 | 2 | Pembrolizumab | Cisplatin | 30 | Pembro + Cis + EBRT | ≥ Grade 3 AE | Best OR, metastasis, salvage RC |
| NCT02891161 | 1, 2 | Durvalumab | None | 42 | Durva + EBRT | DLT, PFS, DCR | CR, OS |
| NCT03993249 | 2 | Nivolumab | Not specified | 78 | CRT + Nivo | LCR | AE, BCFF, OS, QOL |
| CRT + Placebo | |||||||
| NCT03775265 | 3 | Atezolizumab | Gemcitabine | 475 | CRT + Atezo | BIEFS | OS, PFS, MFS, CSS, QOL |
| Cisplatin | CRT + Placebo | ||||||
| Fluorouracil | |||||||
| Mitomycin-C |
AE, adverse events; BCFF, bladder cancer failure-free rate; BIDFS, bladder-intact disease-free survival; BIEFS, bladder intact event-free survival; CSS, cancer-specific survival; DCR, disease control rate; DLT, dose limiting toxicity; LCR, locoregional control rate; MFS, metastasis-free survival; MTD, maximal tolerated dose; OR, objective response; OS, overall survival; PFS, progression free survival; QOL, quality of life.
Conclusions
BPT with contemporary technique provides comparable oncological outcomes and better quality of life when compared to our gold standard RC, suggesting the importance of a multidisciplinary approach in treating MIBC patients. Identification of novel biomarkers will help determine treatment modalities (RC vs. BPT) in MIBC patients. Moreover, these biomarkers could be used to design optimal bladder preservation regimens comprising of consolidative partial cystectomy, molecular agents, hypoxia modifying agents, and immunotherapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marco Moschini) for the Series “Bladder Cancer” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.02.01/coif). The Series “Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- VanderWalde NA, Chi MT, Hurria A, et al. Treatment of muscle invasive bladder cancer in the elderly: navigating the trade-offs of risk and benefit. World J Urol 2016;34:3-11. [Crossref] [PubMed]
-
Muscle-invasive and Metastatic Bladder Cancer - Makino K, Nakagawa T, Kanatani A, et al. Biphasic decline in renal function after radical cystectomy with urinary diversion. Int J Clin Oncol 2017;22:359-65. [Crossref] [PubMed]
- Nishikawa M, Miyake H, Yamashita M, et al. Long-term changes in renal function outcomes following radical cystectomy and urinary diversion. Int J Clin Oncol 2014;19:1105-11. [Crossref] [PubMed]
- Isbarn H, Jeldres C, Zini L, et al. A population based assessment of perioperative mortality after cystectomy for bladder cancer. J Urol 2009;182:70-7. [Crossref] [PubMed]
- Nielsen ME, Mallin K, Weaver MA, et al. Association of hospital volume with conditional 90-day mortality after cystectomy: an analysis of the National Cancer Data Base. BJU Int 2014;114:46-55. [Crossref] [PubMed]
- Fonteyne V, Ost P, Bellmunt J, et al. Curative Treatment for Muscle Invasive Bladder Cancer in Elderly Patients: A Systematic Review. Eur Urol 2018;73:40-50. [Crossref] [PubMed]
- Izquierdo L, Peri L, Leon P, et al. The role of cystectomy in elderly patients - a multicentre analysis. BJU Int 2015;116:73-9. [Crossref] [PubMed]
- Gore JL, Litwin MS, Lai J, et al. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst 2010;102:802-11. [Crossref] [PubMed]
- Gray PJ, Fedewa SA, Shipley WU, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol 2013;63:823-9. [Crossref] [PubMed]
- Herr HW. Transurethral resection of muscle-invasive bladder cancer: 10-year outcome. J Clin Oncol 2001;19:89-93. [Crossref] [PubMed]
- Solsona E, Iborra I, Collado A, et al. Feasibility of radical transurethral resection as monotherapy for selected patients with muscle invasive bladder cancer. J Urol 2010;184:475-80. [Crossref] [PubMed]
- Gospodarowicz MK, Hawkins NV, Rawlings GA, et al. Radical radiotherapy for muscle invasive transitional cell carcinoma of the bladder: failure analysis. J Urol 1989;142:1448-53; discussion 1453-4. [Crossref] [PubMed]
- Mameghan H, Fisher R, Mameghan J, et al. Analysis of failure following definitive radiotherapy for invasive transitional cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys 1995;31:247-54. [Crossref] [PubMed]
- Coppin CM, Gospodarowicz MK, James K, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1996;14:2901-7. [Crossref] [PubMed]
- James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 2012;366:1477-88. [Crossref] [PubMed]
- Kaufman DS, Shipley WU, Griffin PP, et al. Selective bladder preservation by combination treatment of invasive bladder cancer. N Engl J Med 1993;329:1377-82. [Crossref] [PubMed]
- Housset M, Maulard C, Chretien Y, et al. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: a prospective study. J Clin Oncol 1993;11:2150-7. [Crossref] [PubMed]
- Sauer R, Schrott KM, Dunst J, et al. Preliminary results of treatment of invasive bladder carcinoma with radiotherapy and cisplatin. Int J Radiat Oncol Biol Phys 1988;15:871-5. [Crossref] [PubMed]
- Shipley WU, Kaufman DS, Zehr E, et al. Selective bladder preservation by combined modality protocol treatment: long-term outcomes of 190 patients with invasive bladder cancer. Urology 2002;60:62-7; discussion 67-8. [Crossref] [PubMed]
- Rödel C, Grabenbauer GG, Kuhn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol 2002;20:3061-71. [Crossref] [PubMed]
- Tunio MA, Hashmi A, Qayyum A, et al. Whole-pelvis or bladder-only chemoradiation for lymph node-negative invasive bladder cancer: single-institution experience. Int J Radiat Oncol Biol Phys 2012;82:e457-62. [Crossref] [PubMed]
- Weiss C, Engehausen DG, Krause FS, et al. Radiochemotherapy with cisplatin and 5-fluorouracil after transurethral surgery in patients with bladder cancer. Int J Radiat Oncol Biol Phys 2007;68:1072-80. [Crossref] [PubMed]
- Chang SS, Bochner BH, Chou R, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol 2017;198:552-9. [Crossref] [PubMed]
- Available online: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- Koga F, Kihara K, Yoshida S, et al. Selective bladder-sparing protocol consisting of induction low-dose chemoradiotherapy plus partial cystectomy with pelvic lymph node dissection against muscle-invasive bladder cancer: oncological outcomes of the initial 46 patients. BJU Int 2012;109:860-6. [Crossref] [PubMed]
- Chen RC, Shipley WU, Efstathiou JA, et al. Trimodality bladder preservation therapy for muscle-invasive bladder cancer. J Natl Compr Canc Netw 2013;11:952-60. [Crossref] [PubMed]
- Mitin T, George A, Zietman AL, et al. Long-Term Outcomes Among Patients Who Achieve Complete or Near-Complete Responses After the Induction Phase of Bladder-Preserving Combined-Modality Therapy for Muscle-Invasive Bladder Cancer: A Pooled Analysis of NRG Oncology/RTOG 9906 and 0233. Int J Radiat Oncol Biol Phys 2016;94:67-74. [Crossref] [PubMed]
- Ploussard G, Daneshmand S, Efstathiou JA, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol 2014;66:120-37. [Crossref] [PubMed]
- Onozawa M, Miyanaga N, Hinotsu S, et al. Analysis of Intravesical Recurrence After Bladder-preserving Therapy for Muscle-invasive Bladder Cancer. Jpn J Clin Oncol 2012;42:825-30. [Crossref] [PubMed]
- Koga F, Kihara K. Selective bladder preservation with curative intent for muscle-invasive bladder cancer: a contemporary review. Int J Urol 2012;19:388-401. [Crossref] [PubMed]
- Krasnow RE, Drumm M, Roberts HJ, et al. Clinical Outcomes of Patients with Histologic Variants of Urothelial Cancer Treated with Trimodality Bladder-sparing Therapy. Eur Urol 2017;72:54-60. [Crossref] [PubMed]
- Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2013;63:45-57. [Crossref] [PubMed]
- Eswara JR, Efstathiou JA, Heney NM, et al. Complications and long-term results of salvage cystectomy after failed bladder sparing therapy for muscle invasive bladder cancer. J Urol 2012;187:463-8. [Crossref] [PubMed]
- Efstathiou JA, Spiegel DY, Shipley WU, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol 2012;61:705-11. [Crossref] [PubMed]
- Coen JJ, Zhang P, Saylor PJ, et al. Bladder Preservation With Twice-a-Day Radiation Plus Fluorouracil/Cisplatin or Once Daily Radiation Plus Gemcitabine for Muscle-Invasive Bladder Cancer: NRG/RTOG 0712-A Randomized Phase II Trial. J Clin Oncol 2019;37:44-51. [Crossref] [PubMed]
- Caffo O, Fellin G, Graffer U, et al. Phase I study of gemcitabine and radiotherapy plus cisplatin after transurethral resection as conservative treatment for infiltrating bladder cancer. Int J Radiat Oncol Biol Phys 2003;57:1310-6. [Crossref] [PubMed]
- Oh KS, Soto DE, Smith DC, et al. Combined-modality therapy with gemcitabine and radiation therapy as a bladder preservation strategy: long-term results of a phase I trial. Int J Radiat Oncol Biol Phys 2009;74:511-7. [Crossref] [PubMed]
- Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 2006;176:2414-22; discussion 2422. [Crossref] [PubMed]
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19:666-75. [Crossref] [PubMed]
- Cahn DB, Handorf EA, Ghiraldi EM, et al. Contemporary use trends and survival outcomes in patients undergoing radical cystectomy or bladder-preservation therapy for muscle-invasive bladder cancer. Cancer 2017;123:4337-45. [Crossref] [PubMed]
- Ritch CR, Balise R, Prakash NS, et al. Propensity matched comparative analysis of survival following chemoradiation or radical cystectomy for muscle-invasive bladder cancer. BJU Int 2018;121:745-51. [Crossref] [PubMed]
- Kulkarni GS, Hermanns T, Wei Y, et al. Propensity Score Analysis of Radical Cystectomy Versus Bladder-Sparing Trimodal Therapy in the Setting of a Multidisciplinary Bladder Cancer Clinic. J Clin Oncol 2017;35:2299-305. [Crossref] [PubMed]
- Lagrange JL, Bascoul-Mollevi C, Geoffrois L, et al. Quality of life assessment after concurrent chemoradiation for invasive bladder cancer: results of a multicenter prospective study (GETUG 97-015). Int J Radiat Oncol Biol Phys 2011;79:172-8. [Crossref] [PubMed]
- Herman JM, Smith DC, Montie J, et al. Prospective quality-of-life assessment in patients receiving concurrent gemcitabine and radiotherapy as a bladder preservation strategy. Urology 2004;64:69-73. [Crossref] [PubMed]
- Zietman AL, Sacco D, Skowronski U, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: results of a urodynamic and quality of life study on long-term survivors. J Urol 2003;170:1772-6. [Crossref] [PubMed]
- Henningsohn L, Wijkstrom H, Dickman PW, et al. Distressful symptoms after radical radiotherapy for urinary bladder cancer. Radiother Oncol 2002;62:215-25. [Crossref] [PubMed]
- Caffo O, Fellin G, Graffer U, et al. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma. A survey by a self-administered questionnaire. Cancer 1996;78:1089-97. [Crossref] [PubMed]
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017;171:540-56.e25. [PubMed]
- Mouw KW. DNA Repair Pathway Alterations in Bladder Cancer. Cancers (Basel) 2017;9: [Crossref] [PubMed]
- Thompson LH. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: the molecular choreography. Mutat Res 2012;751:158-246. [Crossref] [PubMed]
- Syed A, Tainer JA. The MRE11-RAD50-NBS1 Complex Conducts the Orchestration of Damage Signaling and Outcomes to Stress in DNA Replication and Repair. Annu Rev Biochem 2018;87:263-94. [Crossref] [PubMed]
- Choudhury A, Nelson LD, Teo MT, et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res 2010;70:7017-26. [Crossref] [PubMed]
- Laurberg JR, Brems-Eskildsen AS, Nordentoft I, et al. Expression of TIP60 (tat-interactive protein) and MRE11 (meiotic recombination 11 homolog) predict treatment-specific outcome of localised invasive bladder cancer. BJU Int 2012;110:E1228-36. [Crossref] [PubMed]
- Magliocco AM, Moughan J, Simko J, et al. The impact of MRE11 in nuclear to cytoplasmic ratio on outcomes in muscle invasive bladder cancer an analysis of NRG/RTOG 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2017;35:343. [Crossref]
- Walker AK, Karaszi K, Valentine H, et al. MRE11 as a Predictive Biomarker of Outcome After Radiation Therapy in Bladder Cancer. Int J Radiat Oncol Biol Phys 2019;104:809-18. [Crossref] [PubMed]
- Bellmunt J, Paz-Ares L, Cuello M, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol 2007;18:522-8. [Crossref] [PubMed]
- Sakano S, Ogawa S, Yamamoto Y, et al. ERCC1 and XRCC1 expression predicts survival in bladder cancer patients receiving combined trimodality therapy. Mol Clin Oncol 2013;1:403-10. [Crossref] [PubMed]
- Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol 2015;68:959-67. [Crossref] [PubMed]
- Desai NB, Scott SN, Zabor EC, et al. Genomic characterization of response to chemoradiation in urothelial bladder cancer. Cancer 2016;122:3715-23. [Crossref] [PubMed]
- Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. [Crossref] [PubMed]
- Chakravarti A, Winter K, Wu CL, et al. Expression of the epidermal growth factor receptor and Her-2 are predictors of favorable outcome and reduced complete response rates, respectively, in patients with muscle-invading bladder cancers treated by concurrent radiation and cisplatin-based chemotherapy: a report from the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2005;62:309-17. [Crossref] [PubMed]
- Inoue M, Koga F, Yoshida S, et al. Significance of ERBB2 overexpression in therapeutic resistance and cancer-specific survival in muscle-invasive bladder cancer patients treated with chemoradiation-based selective bladder-sparing approach. Int J Radiat Oncol Biol Phys 2014;90:303-11. [Crossref] [PubMed]
- Zu X, Tang Z, Li Y, et al. Vascular endothelial growth factor-C expression in bladder transitional cell cancer and its relationship to lymph node metastasis. BJU Int 2006;98:1090-3. [Crossref] [PubMed]
- Lautenschlaeger T, George A, Klimowicz AC, et al. Bladder preservation therapy for muscle-invading bladder cancers on Radiation Therapy Oncology Group trials 8802, 8903, 9506, and 9706: vascular endothelial growth factor B overexpression predicts for increased distant metastasis and shorter survival. Oncologist 2013;18:685-6. [Crossref] [PubMed]
- Keck B, Wach S, Taubert H, et al. Neuropilin-2 and its ligand VEGF-C predict treatment response after transurethral resection and radiochemotherapy in bladder cancer patients. Int J Cancer 2015;136:443-51. [Crossref] [PubMed]
- Gerdes J, Lemke H, Baisch H, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984;133:1710-5. [PubMed]
- Rödel C, Grabenbauer GG, Rödel F, et al. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma: possible predictors for response to radiochemotherapy and successful bladder preservation. Int J Radiat Oncol Biol Phys 2000;46:1213-21. [Crossref] [PubMed]
- Tanabe K, Yoshida S, Koga F, et al. High Ki-67 Expression Predicts Favorable Survival in Muscle-Invasive Bladder Cancer Patients Treated With Chemoradiation-Based Bladder-Sparing Protocol. Clin Genitourin Cancer 2015;13:e243-51. [Crossref] [PubMed]
- Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009;11:102-25. [Crossref] [PubMed]
- Yoshida S, Koga F, Kobayashi S, et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys 2012;83:e21-7. [Crossref] [PubMed]
- Matsumoto H, Wada T, Fukunaga K, et al. Bax to Bcl-2 ratio and Ki-67 index are useful predictors of neoadjuvant chemoradiation therapy in bladder cancer. Jpn J Clin Oncol 2004;34:124-30. [Crossref] [PubMed]
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004;4:437-47. [Crossref] [PubMed]
- Hoskin PJ, Rojas AM, Bentzen SM, et al. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol 2010;28:4912-8. [Crossref] [PubMed]
- Yang L, Taylor J, Eustace A, et al. A Gene Signature for Selecting Benefit from Hypoxia Modification of Radiotherapy for High-Risk Bladder Cancer Patients. Clin Cancer Res 2017;23:4761-8. [Crossref] [PubMed]
- McConkey DJ, Choi W, Shen Y, et al. A Prognostic Gene Expression Signature in the Molecular Classification of Chemotherapy-naive Urothelial Cancer is Predictive of Clinical Outcomes from Neoadjuvant Chemotherapy: A Phase 2 Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin with Bevacizumab in Urothelial Cancer. Eur Urol 2016;69:855-62. [Crossref] [PubMed]
- Miyamoto DT, Gibb E, Mouw KW, et al. Genomic profiling of muscle invasive bladder cancer to predict response to bladder-sparing trimodality therapy. J Clin Oncol 2018;36:513. [Crossref]
- Sjödahl G, Lovgren K, Lauss M, et al. Toward a molecular pathologic classification of urothelial carcinoma. Am J Pathol 2013;183:681-91. [Crossref] [PubMed]
- Tanaka H, Yoshida S, Koga F, et al. Impact of Immunohistochemistry-Based Subtypes in Muscle-Invasive Bladder Cancer on Response to Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2018;102:1408-16. [Crossref] [PubMed]
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66. [Crossref] [PubMed]
- Sabaa MA, El-Gamal OM, Abo-Elenen M, et al. Combined modality treatment with bladder preservation for muscle invasive bladder cancer. Urol Oncol 2010;28:14-20. [Crossref] [PubMed]
- Jiang DM, Jiang H, Chung PWM, et al. Neoadjuvant Chemotherapy Before Bladder-Sparing Chemoradiotherapy in Patients With Nonmetastatic Muscle-Invasive Bladder Cancer. Clin Genitourin Cancer 2019;17:38-45. [Crossref] [PubMed]
- Kaufman DS, Winter KA, Shipley WU, et al. Phase I-II RTOG study (99-06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology 2009;73:833-7. [Crossref] [PubMed]
- Mitin T, Hunt D, Shipley WU, et al. Transurethral surgery and twice-daily radiation plus paclitaxel-cisplatin or fluorouracil-cisplatin with selective bladder preservation and adjuvant chemotherapy for patients with muscle invasive bladder cancer (RTOG 0233): a randomised multicentre phase 2 trial. Lancet Oncol 2013;14:863-72. [Crossref] [PubMed]
- Kijima T, Tanaka H, Koga F, et al. Selective tetramodal bladder-preservation therapy, incorporating induction chemoradiotherapy and consolidative partial cystectomy with pelvic lymph node dissection for muscle-invasive bladder cancer: oncological and functional outcomes of 107 patients. BJU Int 2019;124:242-50. [Crossref] [PubMed]
- Toide M, Yokoyama M, Fujiwara M, et al. Renal function after bladder-preserving therapy for patients with muscle-invasive bladder cancer: Results of selective bladder-preserving tetramodality therapy consisting of maximal transurethral resection, induction chemoradiotherapy and partial cystectomy. Int J Urol 2019;26:1010-2. [Crossref] [PubMed]
- Buchwald ZS, Efstathiou JA. Immunotherapy and Radiation - A New Combined Treatment Approach for Bladder Cancer? Bladder Cancer 2015;1:15-27. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Mouw KW, Goldberg MS, Konstantinopoulos PA, et al. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov 2017;7:675-93. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Galsky MD, Wang H, Hahn NM, et al. Phase 2 Trial of Gemcitabine, Cisplatin, plus Ipilimumab in Patients with Metastatic Urothelial Cancer and Impact of DNA Damage Response Gene Mutations on Outcomes. Eur Urol 2018;73:751-9. [Crossref] [PubMed]
Cite this article as: Tanaka H, Kijima T, Fujii Y. Bladder preservation therapy in muscle-invasive bladder cancer: Current evidence and future perspectives. AME Med J 2020;5:16.

