
A narrative review of redo coronary artery bypass grafting
Introduction
Coronary artery bypass grafting (CABG) continues to be the treatment of choice in patients with complex multivessel coronary artery disease (CAD) (1-3) and offers long term palliation. The evolution of surgical coronary revascularization over recent decades has been well recognized and re-operative CABG is being performed more and more infrequently in contemporary practice (4,5). Redo CABG is warranted when patients with a prior surgical myocardial revascularization have progression of their native CAD or atherosclerosis of graft(s) leading to stenosis or occlusion and development of symptoms that limit their regular activity. The morbidity and mortality following redo CABG is higher due to the increased intraoperative complexity (6). The challenges of redo CABG include decision making aspects, difficult technical operation, shortage of conduits, sicker patients apart from the problems of re-entrant sternotomy (7-9). Patients requiring redo CABG includes those with either failure of all conduits or failure of vein grafts with patent arterial conduits or the reverse combination. With the increasing longevity of CABG patients, re-operative CABG surgery has emerged as an essential requisite in the cardiac surgeon’s armamentarium (10). Despite treating patients with more complex CAD and greater medical co-morbidities, improvements have occurred in operative strategies and techniques that have resulted in a reduction of operative morbidity and mortality in this challenging population (11). Traditionally, coronary reoperations have been performed using cardiopulmonary bypass (CPB) with the aorta cross clamped and heart arrested using cardioplegia. But this cannot be the norm in redo CABG since many in this high-risk subgroup are generally elderly who are frequently frail and have a diminished physiologic reserve when compared to the young (9,12,13). This review describes the changing trends in the incidence of redo CABG, risk profile and challenges associated with redo CABG indications, preoperative evaluation and patient selection and outcomes of redo CABG. This review also describes various strategies to improve surgical outcomes and prospects of redo CABG. A literature review focused on the epidemiology, evaluation, operative techniques and strategies, and outcomes associated with reoperative CABG. I present this article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-20-50/rc).
Incidence of redo CABG
The frequency of redo CABG procedures has been decreasing steadily over time, yet redo CABG forms an important part of the surgical coronary revascularization workload (4,14). Of all the CABG procedures reported to the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database, as a percentage of overall CABG volume, redo CABG decreased from 6.4% (8,820/137,267) in 2000 to 3.6% (5,734/160,997) in 2009, a 35% reduction in redo CABG volume over 10 years (4). In 2017 the proportion of redo CABG was reported to be 2% (4). According to data from the annual report of the Japanese Association for Thoracic Surgery (JATS), the ratio of redo CABG to the total number of CABG procedures has decreased from 10% to 2% over 10 years (15,16). A need for repeat revascularization of 2% at the end of the first year after primary CABG, 7% at 5 years, 13% at 10 years and 16% at 18 years had been reported in the study by the STS accounting for 723,134 patients who had undergone a prior CABG, even though the point incidence of redo CABG was as low as 0.1%, 0.6%, 1.3% and 1.7% respectively (5). Different studies have corroborated the fact that there is a year on year increase in the incidence of redo procedures ranging from <3% at 5 years, up to 10% at the end of 10 years and rising to 20–30% near about 15 years after the first revascularization surgery (17-19). When viewed in terms of success of the primary procedure, 73% of patients were free from re-intervention at 15 years, 60% at 20 years and 46% at 25 years among the 26,927 patients followed up at the Cleveland Clinic as reported by Sabik et al. (14), implying that nearly one in every two patients who undergoes a primary CABG would need repeat re-intervention after 25 years.
van Domburg and colleagues in a 30-year follow-up of over 1,041 primary CABG procedures wherein all venous conduits were used, reported re-interventions in 36% of the patients; 29.6% had redo CABG. However, it was noted that percutaneous coronary intervention (PCI) was the re-intervention in those 20 years after the primary CABG (20). Redo CABG currently accounts for 4% of all CABG procedures in the UK and India (21,22). Higher prevalence of repeat revascularization was associated with female sex, severe CAD, preoperative dialysis and incomplete revascularization. The proportion of CABG patients needing a redo procedure has been consistently declining largely due to increasing adoption of the left internal mammary artery (LIMA) for tackling lesions in the left anterior descending artery (LAD), use of additional arterial grafts during primary operation, percutaneous coronary intervention and improvement in postoperative medical therapy for secondary prevention (16). Further left main disease, smoking and advanced age were associated with the lower requirement for repeat revascularization (5).
The risk profile of patients undergoing redo CABG
There is more extensive CAD and a more compromised left ventricular function among the patients who required redo CABG with older age being another risk factor (9,16). Globally, the patient risk has worsened overtime with more patients who had a prior percutaneous coronary intervention (PCI) presenting with acute coronary syndrome and heart failure in need of an urgent or emergent surgery. These patients have a greater prevalence of other co-morbidities such as diabetes, hypertension, and chronic kidney disease (9,16). A study of the STS database compared the demographics of patients who underwent redo CABG patients in 2009 with those in 2000 and found no significant change in age or gender. There were more co-morbidities in more severe forms of congestive cardiac failure, left main CAD and myocardial infarction among the redo patients in the year 2009 (4). A similar worsening of the preoperative risk factors for redo CABG was reported over twenty years, from 1990 to 2009, by Spiliotopoulos and colleagues (23). Yap et al. (24) and Sabik et al. (11) have shown that the risk matrix was significantly worse for redo CABG when compared to those undergoing a primary procedure. In India patients undergoing primary CABG are at a younger age than their Western counterparts in need of redo CABG (25).
Challenges of redo CABG
There are ample technical challenges during a redo CABG (Table 1). The shortage of conduits is frequently encountered due to their use at the previous operation (9). Currently given the popularity of endoscopic vein harvest techniques, ultrasonic evaluation of saphenous vein is needed to ascertain the extent of the remnant saphenous vein. Cardiovascular injury on sternal re-entry is an ever-present danger as the pericardium has already been breached and could result in cardiac injury, damage to the great vessels and bypass grafts from an undue proximity or adherence to the sternum. A diseased aorta enveloped in scar tissue cramps the space needed for cannulation and aortic clamping while placing the patient on pump support additionally renders proximal anastomoses of the venous grafts more challenging. Effective delivery of cardioplegia to all areas of the myocardium is made difficult when the native coronary arteries and bypass grafts are occluded. A patent internal mammary artery (IMA) graft to a totally occluded LAD artery is not uncommon and protection of the patent IMA may be quite challenging (8). It is reported that 40% of the patients who had intraoperative injury to the LIMA graft during a redo CABG had a significant perioperative myocardial infarction. Identifying and exposing the earlier internal thoracic artery grafts is often quite difficult in redo CABG cases and entails a higher risk of graft injury (9,26). Vein graft atherosclerosis is an important predisposing factor for the redo procedure. Ongoing antegrade flow could be encountered when the atherosclerotic vein grafts are partially occluded which in turn puts the patient at risk for embolization and myocardial infarction (7).
Table 1
| Shortage of conduits |
| Cardiovascular injury on re-entry |
| Patent right ITA graft crossing midline at risk of injury |
| Potentially diseased aorta and surrounded with scar tissue with limited space to cannulate for CPB, clamp, and proximal anastomosis(es) |
| Myocardial protection issues as effective delivery of cardioplegia to all areas of the myocardium can be challenging in the presence of occluded coronaries and grafts |
| A patent ITA to a totally occluded LAD |
| Higher usage of IABP support (more than four times that of primary CABG) |
| Difficulties in achieving optimal myocardial protection and complete revascularization |
| Difficulty with finding and controlling patent ITA grafts |
| ITA graft injuries have serious consequences and patent ITAs are particularly vulnerable |
| Finding a coronary target can be challenging |
| The quality of targets may be poor |
| More bleeding because of adhesions |
| Antithrombotic therapy in the immediate preoperative period |
| Partially occluded atherosclerotic veins that have continued antegrade flow are predisposed to embolization leading to fatal MI |
CABG, coronary artery bypass grafting; IABP, intra-aortic balloon pump; ITA, internal thoracic artery; LAD, left anterior descending artery; MI, myocardial infarction.
The availability of arterial conduits is compromised if the IMA graft was also used at primary CABG. Usage of radial artery, right gastroepiploic artery, other IMA graft may be required and is associated with greater technical difficulty and increased surgical time. Difficulty in visualization and accessing the coronary targets, and heightened risk of coronary microembolization may lead to myocardial infarction that increases morbidity and mortality of coronary reoperations.
Indications for reoperation
The nonavailability of functional patent arterial grafts is a key deciding factor (27). Patients with suspected early graft dysfunction either in the form of ischemic symptoms or raised cardiac enzymes or new regional wall motion abnormalities should undergo angiogram followed by appropriate revascularization by PCI or redo CABG. The late graft dysfunction or progression of disease in the form of severe symptoms despite optimal management or large area of inducible ischemia by noninvasive evaluation should undergo angiogram followed by either PCI or redo CABG (28-36). The mortality risk doubles or even quadruples for redo CABG in comparison with primary CABG at centers where redo surgeries are performed infrequently (5,11,24,37). There is more to be understood regarding the efficacy of PCI and redo CABG in repeat revascularization. The distribution of patients for revascularization by PCI, redo CABG or noninvasive medical management differs significantly in the peer-reviewed literature; a few favoring PCI others favoring redo CABG (27,38). The overall three-year mortality was similar between repeat CABG and revascularization through PCI as reported in the AWESOME randomized control trial and registry (38,39). Two other studies also reported a similar incidence of mortality and myocardial infarction after redo CABG and PCI as secondary intervention, while there appeared to be a preference for PCI for revascularization (40). PCI has gained an edge for revascularization whenever there is an amenable target vessel anatomy since there is a higher risk of operative mortality with redo CABG, while the long-term results are comparable (40).
Management of reoperative CABG patients
Globally, over the last couple of decades, great strides have been made in the perioperative management of redo CABG patients. These improvements include technological developments as well as medical and surgical innovations in imaging and management of the patients undergoing redo CABG. Preoperative planning has been strengthened with the effective use of advanced computerized tomographic imaging techniques (41). The use of intraoperative transesophageal echocardiography and epiaortic scanning has facilitated the placement of cannulae for CPB and retrograde cardioplegia (42). While the overall rate of performing redo CABG has shown a decline, there is a marked increase in the rates of risk factors among this subset of patients (43).
Strategies to improve outcomes of redo CABG (Table 2)
Table 2
| Area of concern | Strategic approach |
|---|---|
| Operative planning & team strategy | Thorough patient evaluation, careful review of preoperative imaging studies and operative planning with the team |
| CT chest to delineate previous grafts, assess quality of aorta and determine distance of heart from back of sternum | |
| Evaluation of anatomy and patency of axillary, iliac, and femoral vessels should the need for extrathoracic cannulation arise | |
| Sternotomy & essentials of local dissection | A sternotomy is preferably performed with an oscillating saw and identifying the pericardial edges and intrapericardial dissection in the right plane |
| In extreme-risk cases, sternotomy after instituting peripheral cardiopulmonary bypass | |
| Early dissection of the aorta and the right side of the heart is performed to allow for central cannulation for CPB and epiaortic ultrasound to locate disease-free spots in the aorta for cannulation and clamping | |
| Dissection of the left side of the heart and a patent LITA graft may be performed before going on CPB if the dissection is easy and safe | |
| The no-touch method for old venous grafts | |
| Management of intraoperative complications & myocardial protection | If injury or ischemia occurs, the prime objectives are to protect the brain and heart and this often requires emergency cannulation with or without hypothermia |
| Perfusion or cardioplegia to the injured cardiac territory and retrograde delivery of cardioplegia if needed | |
| Control of a patent ITA graft while delivering cardioplegia, and primary repair of injured bypass grafts should be backed up with a replacement graft | |
| Grafting configuration & choice of bypass conduits | Arterial grafts should be used whenever possible, especially in younger patients and those with a reasonable life expectancy |
| LITA-to-LAD confers a survival advantage | |
| RITA or RA to be used in whom the LITA has been previously used | |
| Composite Y or T grafts can extend the reach of the grafts (free RITA can be taken off the patent in-situ LITA-to-LAD graft to bypass a lateral wall target) | |
| Off-pump technique | Eliminates CPB-induced complications—coagulopathy, avoidance of aortic manipulation |
| Base new grafts on ITA inflow to avoid aortic manipulation | |
| Use of intracoronary shunts offers effective myocardial protection | |
| On-pump technique | Redo CABG with the use of CPB is associated with lesser perioperative mortality even in hemodynamically unstable patients |
| Sternal-sparing approaches | In patients with a patent LITA-to-LAD graft and significant myocardial ischemia in a sizable non-LAD territory not amenable to percutaneous intervention, sternal-sparing thoracotomy approach with or without cardiopulmonary bypass is a less invasive option |
| Grafting strategies | Customized arterial grafting strategies using RITA and RA grafts may be advantageous |
| Postoperative care | Routine post-redo CABG care includes guideline-directed medical therapy |
| Secondary preventive strategies to optimize short- and long-term outcomes |
CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; LAD, left anterior descending artery; LITA, left internal thoracic artery; RA, radial artery; RITA, right internal thoracic artery.
Operative planning and team strategy for revascularization
To improve the surgical outcomes a thorough preoperative evaluation of the patients and strategic operative planning are prerequisites to minimize the intraoperative complications (44). A team briefing about the plan of the procedure, the anticipated adverse events and the strategy to tackle the difficult situation is the cornerstone of operative planning. A careful review of coronary angiograms and computed tomographic imaging of the chest is mandatory. Patent IMAs are particularly valuable and injury to the IMAs should be avoided.
Use of imaging modalities
A pre-operative CT scan is helpful to evaluate the space between the back of the sternum and its posteriorly-related vital structures. One can avoid unpleasant surprises by establishing the patency and anatomy of the femoral, iliac and axillary vessels using Doppler ultrasonography should there be a need for peripheral cannulation (9).
Sternal re-entry
The use of an oscillating saw (Figure 1) has been shown to promote an uneventful sternal re-entry with further safety to the retrosternal structures being ensured with the use of a micro-oscillating saw (45,46). The wires are cut anteriorly and bent back and retained in situ on the posterior aspect of the sternum. If severe adhesions are encountered an extra-cardiac access via the femoral artery or the right axillary artery is essential for initiating CPB.
Lysis of adhesions and essentials of local dissection
After achieving sternal entry, it is usual to secure the easier part of the dissection by commencing at the inferior part of the heart. This is followed by establishing the arterial and venous accesses by proceeding next with dissecting the ascending aorta and the right atrium thereafter.
In a conventional approach, this would be the time to institute CPB and continue further dissection in the comfort of the decompressed heart. Epiaortic ultrasonography may help to demarcate segments that are free of disease in the aorta for cannulation, clamping and proximal anastomoses. In the case of an off-pump redo CABG procedure, the dissecting planes can be visualized better by rotating the heart using a heart positioner. However, it is important to complete the dissection of the IMA along its entire length as this is essential to allow for the rotation of the heart during off-pump surgery (Figure 2). A harmonic scalpel may be useful in minimizing the bleeding during the lysis of pericardial adhesions. An upward dissection from the cardiac apex in a plane close to the pericardium would help locate the proximally placed patent LIMA underneath the lung. A harmonic scalpel enhanced dissection of the IMA graft is a strong recommendation (47). No-touch method of dissection of diseased vein grafts should be adopted to avoid embolization of the grafts.
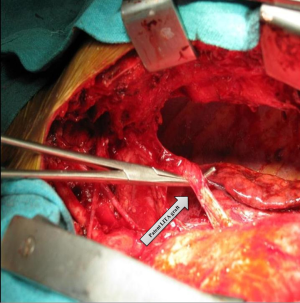
The IMA graft needs to be clamped across using an atraumatic clamp during the delivery of cardioplegia. If the right IMA (RIMA) grafted to the LAD is patent, it would need to be dissected free before aortic cannulation. This would ease the placement of the partial occlusion clamp while performing the proximal anastomosis of the intended grafts, whether arterial or venous. The entire dissection has to be intra-pericardial. Waiting until the aortic cross-clamping and cardiac arrest is established to dissect out the left ventricle is advantageous since dissection is rendered more comfortable and proves more accurate. It is also safer since there is less damage to the epicardium and less bleeding.
Myocardial protection
Myocardial protection is extremely important, especially in redo CABG, because the coronary arteries of patients undergoing redo CABG are often severely atherosclerotic, and occluded. Care should be taken to protect the myocardium, especially if it is mainly perfused by the IMA. In such a case, the cardioplegic solution should be given into the root of the aorta and also through the coronary sinus in a retrograde fashion.
Management of patent diseased vein grafts
Vein grafts to the LAD territory which are diseased but patent pose a dilemma since it has been shown the replacement of a patent but diseased vein graft with an arterial graft has a higher chance of hypoperfusion. This is because the flow through the new LIMA graft is lower than that of the stenosed vein graft. Hence diseased vein grafts that are not critically stenosed are better left undisturbed (48). A skeletonized IMA has been shown to have superior flow to that of a pedicled IMA graft and this may be a better option when replacing the patent diseased vein graft is a must (49).
Managing intraoperative complications
Most complications stem from injury and/or ischemia and in both situations preserving and protecting cardiac and neural function is paramount. These situations warrant an immediate cannulation and initiation of CPB without hypothermia. A primary repair of the injured grafts must be attempted to restore perfusion to the territory at risk. Once the crisis is averted, the injured graft which had been repaired may need to be replaced with a new graft in the later part of the surgery. Perfusion of the deprived territory can also be enhanced by delivering cardioplegia through the coronary sinus in a retrograde manner and clamping of a patent IMA graft (9).
Grafting configurations and choice of bypass conduits
The selection of bypass conduits for reoperation CABG is a challenging issue. Broadly, the concepts for the choice of conduit remains the same for both primary and redo CABG. More number of arterial grafts (IMAs, radial artery, right gastroepiploic artery) should be used when there is a higher life expectancy especially younger patients. If only saphenous vein grafts were used at the primary operation, the LIMA and RIMA are a preferred option for redo CABG. Many patients who undergo redo CABG may have a patent IMA-to-LAD graft, while occluded venous grafts to left circumflex arteries and right coronary artery territories. In such cases, replacement of old vein grafts by new saphenous vein grafts to right and left circumflex arteries may be carried out; other choices of conduits are the RIMA and radial artery grafts. Careful selection of conduits and target vessels is needed to avoid competitive flow. Graft spasm remains an important consideration with radial artery graft. When considering the revascularization of the circumflex artery territory, RIMA via the transverse sinus can be a good option, with the added caution that this could be a difficult dissection. In situations where in-situ RIMA is not long enough to reach the target, the single IMA graft arrangement can be upgraded to a bilateral IMA graft arrangement using the RIMA as a free graft (50). Min et al. (51) reported complete revascularization using a free RIMA graft as a side branch from the previous patent LIMA-to-LAD graft. Such graft arrangements using bilateral IMAs may have a better survival, similar to primary CABG (52). For patients in whom LIMA has been used previously harvesting RIMA does not increase the risk of deep sternal wound infections (53). The evidence that the use of at least one IMA graft improves outcomes in the primary CABG is compelling and the same philosophy applies to redo CABG. The right gastroepiploic artery graft may be a good option for a graft to the posterior descending artery in redo CABG (54,55). Gastroepiploic artery grafts are sensitive to competitive flow with concerns related to graft length, size variation and vulnerability to spasm being some of the other challenges (55).
Choice of technique: on-pump versus off-pump
Off-pump CABG is promoted more often in Asian countries. Patients suitable for on-pump redo CABG may also be suitable for off-pump redo CABG and there is no absolute contraindication for reoperative off-pump CABG. There are several reasons favoring off-pump redo CABG. It avoids cannulation of the aorta as well as aortic manipulation in those instances where the IMA inflows feed the prospective new grafts. Further use of intracoronary shunts in off-pump reoperations eliminates concerns related to the effectiveness of cardioplegia. The patients with stable hemodynamics, large epicardial target coronary arteries and anterior, inferior wall coronary targets are suitable for off-pump CABG. However, the off-pump technique is relatively difficult to adopt in patients with dilated heart, mitral insufficiency, lateral wall targets, and patent IMA grafts. Surgical delineation of the target vessels tends to be challenging with off-pump when there is a large heart with excessive epicardial fat. Performing repeat revascularization on beating heart may result in hemodynamic instability.
Maltais et al. reported 6% perioperative mortality in a cohort of patients who all underwent redo CABG using on-pump technique (56). On the other hand, the mortality of redo CABG at 1 month with off-pump technique has been reported to be less than half that of the on-pump technique (57). A meta-analysis of 12 studies found that off-pump redo CABG had a lower mortality in the early postoperative period in selected patients (58). Mishra et al. (12) reported their 10-year experience for a cohort of 296 patients with single and multi-vessel reoperative off-pump CABG with a lower early mortality of 3.5% compared to 5.5%, for on-pump redo CABG (P=0.066). Sajja et al. reported a mortality of 4.4% for a cohort of 68 young patients who underwent redo CABG using on-pump technique (25). Taggart et al. reported 1% hospital mortality for 159 consecutive redo patients over 6 years (59). Several studies have firmly established that there is no difference in in-hospital mortality between on- and off-pump redo CABG cases (60-63). This, however, changes when there are coexisting comorbidities as off-pump CABG has the advantage of a lower in-hospital mortality (64). Additionally, it has been shown that the occurrence of serious complications such as stroke is lower with the off-pump technique (65,66). The incidence of serious adverse effects and operative mortality was statistically lesser in redo surgeries done off-pump as per the propensity analysis done on the Japanese Cardiovascular Surgery Database (67).
Sternal sparing techniques
Although most reoperations are performed via re-entrant median sternotomy and use of CPB, non-sternotomy small incisions with off-pump techniques become handy during reoperations. This approach, with or without CPB, may also be a better option, when there is a LIMA to LAD graft which is stenosed but not suitable for PCI, to address extensive ischemia of the LAD territory. With sternal sparing options there is a reduction of injury to cardiovascular structures, and avoidance of manipulation of the ascending aorta particularly when it is calcified or diseased. Reoperations in situations with limited areas of ischemic myocardium needing revascularization often can be accomplished via a limited incision and without the use of CPB. The distal LAD artery may be exposed via a small anterior thoracotomy. A posterior thoracotomy is a useful option in redo CABG when targeting the lateral wall of the left ventricle (68). Newer, stabilizing devices are usually employed for anastomotic reconstruction, although intra-pericardial adhesions provide some stability. However, a median sternotomy may be useful when harvesting a RIMA graft.
Re-redo coronary artery bypass operations
The CABG procedure either primary or redo is technically similar. Many patients undergoing second- or third-redo CABG usually had their first CABG procedure more than a decade and a half ago and have severe native vessel disease. The primary concern in the re-redo coronary operation is the non-availability of suitable conduits. Currently, re-redo operations are performed infrequently. The operative mortality for the re-reoperations has decreased to 0.8% from 8% (12).
Surgical outcomes of redo CABG
It is reassuring to witness the steady decline in the surgical mortality of reoperative CABG over time from 6% in 2000 to 4.6% in 2009, despite the ever-increasing comorbidities in this group (4). The risk model of the STS nonetheless emphasizes the higher odds ratio of perioperative mortality in redo CABG besides showing an increased incidence of other major complications including prolonged postoperative ventilation and renal failure (9,69). The outcomes of redo CABG are underscored by two important determinants—patient risk profile and surgical experience in reoperative CABG. The challenges of technical difficulties in redo CABG have been surmounted with increasing expertise. Surgical centers with high volumes of redo CABG do not consider redo surgery as a major concern and focus more on the risk profile of the patients with perioperative mortality less than 2%. Table 3 summarizes the incidence of redo CABG and related mortality (70).
Table 3
| Study | No of redo CABGs | Period | Percentage of redo CABGs to overall CABGs | Early mortality of redo CABG |
|---|---|---|---|---|
| Weintraub et al. (6) | 2,030 | 1975–1993 | – | 7% |
| Yau et al. (43) | 1,230 | 1982–1997 | 6% | 6.80% |
| Taggart et al. (59) | 157 | 1987–1992 | – | 1% |
| Lytle et al. (70) | 1,663 | 1988–1991 | – | 3.70% |
| Spiliotopoulos et al. (23) | 1,204 | 1990–2009 | 7.2% (1990–1994) | 4.7% (1990–1999) |
| 2.2% (2005–2009) | 3.8% (2000–2009) | |||
| Sabik et al. (11) | 4,518 | 1990–2003 | 21% | 4.40% |
| Di Mauro et al. (71) | 239 | 1994–2001 | 6.30% | 4.20% |
| Mishra et al. (12) | 538 | 1996–2005 | – | 3.3% (OPCAB) |
| – | 5.8% (on-pump CABG) | |||
| Sajja et al. (25) | 68 | 1998–2004 | 4.60% | 4.40% |
| Machiraju et al. (8) | 543 | 1999–2004 | – | 3% |
| Ghanta et al. (4) | 72,322 | 2000–2009 | 6% (2000) | 6.1% (2000) |
| 3.4% (2009) | 4.6% (2009) | |||
| Yap et al. (24) | 458 | 2001–2008 | 3.40% | 4.80% |
CABG, coronary artery bypass grafting; OPCAB, off-pump coronary artery bypass.
Completeness of revascularization in redo CABG
The goal of reoperation CABG mirrors that of primary CABG which is to achieve complete revascularization. Di Mauro et al. (71) have shown that the incidence of incomplete revascularization in off-pump redo CABG is 17.1% as compared to 5.9% in on-pump redo surgery (P<0.1). Incomplete revascularization is an independent risk factor for cardiac mortality at 5 years. Tugtekin and associates used index of completeness of revascularization to compare on-pump redo CABG and off-pump redo CABG (60) and observed a higher incidence of incomplete revascularization in on-pump redo CABG (56.09% vs. 48.6%, P<0.01).
Prospects
The adoption of multiple arterial grafting techniques during primary CABG has the potential to obviate the need for a redo CABG thereby bringing a further reduction in the need for redo CABG in the future. However, should the need arise, redo CABG would continue to be challenging because patients would in all likelihood have more comorbidities with more complex coronary anatomy and left ventricular dysfunction. The future of redo CABG would benefit from an increased adoption of minimally invasive approaches avoiding sternal re-entry and without the use of CPB. A combination of PCI and off-pump CABG resulting in hybrid revascularization would give the patient “the best of both worlds”.
Conclusions
Redo CABG is a complex operation and appropriate planning and meticulous surgical technique are essential for achieving optimal outcomes. Surgeons who perform redo CABG procedures in a wide spectrum of anatomical situations may find either off-pump or on-pump strategy as helpful. There is a decreasing trend in the frequency of reoperative CABG, which only heightens the challenges of being adept in handling this highly complex surgical procedure. Surgical centers with experienced surgeons have rendered the mortality rate of reoperation CABG akin to that of primary CABG.
Acknowledgments
We thank Dr. Antony L. Basile M.S., F.M.R.F., Medical Director, Star Hospitals, Hyderabad, India and B. Prashanthi M.Sc. (Clinical Research), Clinical Coordinator, Sajja Heart Foundation, Hyderabad, India for the help in preparing the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Shahzad G. Raja) for the series “Coronary Artery Bypass Grafting” published in AME Medical Journal. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-20-50/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-50/coif). The series “Coronary Artery Bypass Grafting” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yusuf S, Zucker D, Chalmers TC. Ten-year results of the randomized control trials of coronary artery bypass graft surgery: tabular data compiled by the collaborative effort of the original trial investigators. Part 1 of 2. Online J Curr Clin Trials 1994;Doc No 145:[3987 words; 38 paragraphs].
- Hannan EL, Wu C, Walford G, et al. Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med 2008;358:331-41. [Crossref] [PubMed]
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. [Crossref] [PubMed]
- Ghanta RK, Kaneko T, Gammie JS, et al. Evolving trends of reoperative coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. J Thorac Cardiovasc Surg 2013;145:364-72. [Crossref] [PubMed]
- Fosbøl EL, Zhao Y, Shahian DM, et al. Repeat coronary revascularization after coronary artery bypass surgery in older adults: the Society of Thoracic Surgeons' national experience, 1991-2007. Circulation 2013;127:1656-63. [Crossref] [PubMed]
- Weintraub WS, Jones EL, Craver JM, et al. In-hospital and long-term outcome after reoperative coronary artery bypass graft surgery. Circulation 1995;92:II50-7. [Crossref] [PubMed]
- Loop FD, Lytle BW, Gill CC, et al. Trends in selection and results of coronary artery reoperations. Ann Thorac Surg 1983;36:380-8. [Crossref] [PubMed]
- Machiraju VR. How to avoid problems in redo coronary artery bypass surgery. J Card Surg 2004;19:284-90. [Crossref] [PubMed]
- Bakaeen FG, Akras Z, Sevsson LG. Redo coronary artery bypass grafting. Indian J Thorac Cardiovasc Surg 2018;34:272-8. [Crossref] [PubMed]
- Ramlawi B, Bedeir K, Garcia-Morales L, et al. Improved short-term outcomes with off-pump reoperative coronary artery bypass grafting. Innovations (Phila) 2014;9:49-53. [Crossref] [PubMed]
- Sabik JF III, Blackstone EH, Houghtaling PL, et al. Is reoperation still a risk factor in coronary artery bypass surgery? Ann Thorac Surg 2005;80:1719-27. [Crossref] [PubMed]
- Mishra YK, Collison SA, Malhotra R, et al. Ten-year experience with single-vessel and multivessel reoperative off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg 2008;135:527-32. [Crossref] [PubMed]
- Bergsland J, Hasnain S, Lajos TZ, et al. Elimination of cardiopulmonary bypass: a prime goal in reoperative coronary bypass surgery. Eur J Cardiothorac Surg 1998;14:59-62; discussion 62-3. [Crossref] [PubMed]
- Sabik JF 3rd, Blackstone EH, Gillinov AM, et al. Occurrence and risk factors for reintervention after coronary artery bypass grafting. Circulation 2006;114:I454-60. [Crossref] [PubMed]
- Amano J, Kuwano H, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2011. Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2013;61:578-607. [Crossref] [PubMed]
- Yaku H, Doi K. Redo coronary artery bypass grafting. Gen Thorac Cardiovasc Surg 2014;62:453-60. [Crossref] [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Reoperation for coronary atherosclerosis. Changing practice in 2509 consecutive patients. Ann Surg 1990;212:378-85. [Crossref] [PubMed]
- Weintraub WS, Jones EL, Craver JM, et al. Frequency of repeat coronary bypass or coronary angioplasty after coronary artery bypass surgery using saphenous venous grafts. Am J Cardiol 1994;73:103-12. [Crossref] [PubMed]
- Kaul TK, Fields BL, Wyatt DA, et al. Reoperative coronary artery bypass surgery: early and late results and management in 1300 patients. J Cardiovasc Surg (Torino) 1995;36:303-12. [PubMed]
- van Domburg RT, Kappetein AP, Bogers AJ. The clinical outcome after coronary bypass surgery: a 30-year follow-up study. Eur Heart J 2009;30:453-8. [Crossref] [PubMed]
- UK Cardiac Surgical Register 1994/5. London: Society of Cardiothoracic Surgeons of Great Britain and Ireland, 1995.
- Sajja LR. The journey of surgery for coronary artery disease in India: adoption, customization, and innovation. Indian J Thorac Cardiovasc Surg 2014;30:116-28. [Crossref]
- Spiliotopoulos K, Maganti M, Brister S, et al. Changing pattern of re-operative coronary artery bypass grafting: A 20-year study. Ann Thorac Surg 2011;92:40-6. [Crossref] [PubMed]
- Yap CH, Sposato L, Akowuah E, et al. Contemporary results show repeat coronary artery bypass grafting remains a risk factor for operative mortality. Ann Thorac Surg 2009;87:1386-91. [Crossref] [PubMed]
- Sajja LR, Mannam GC, Pantula NR, et al. Reoperation for coronary artery disease in the young: early and mid-term results. Indian J Thorac Cardiovasc Surg 2005;21:199-203. [Crossref]
- Gillinov AM, Casselman FP, Lytle BW, et al. Injury to a patent left internal thoracic artery graft at coronary reoperation. Ann Thorac Surg 1999;67:382-6. [Crossref] [PubMed]
- Brener SJ, Lytle BW, Casserly IP, et al. Predictors of revascularization method and long-term outcome of percutaneous coronary intervention or repeat coronary bypass surgery in patients with multivessel coronary disease and previous coronary bypass surgery. Eur Heart J 2006;27:413-8. [Crossref] [PubMed]
- Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery. Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2010;38:S1-52. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- De Bruyne B, Pijls NH, Kalesan B, et al. FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991-1001. Erratum in: N Engl J Med 2012 Nov;367(18):1768. Mobius-Winckler, Sven [corrected to Möbius-Winkler, Sven]. [Crossref] [PubMed]
- Hachamovitch R, Hayes SW, Friedman JD, et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single-photon emission computed tomography. Circulation 2003;107:2900-7. [Crossref] [PubMed]
- Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012-24. [Crossref] [PubMed]
- Sabik JF III, Raza S, Blackstone EH, et al. Value of internal thoracic artery grafting to the left anterior descending coronary artery at coronary reoperation. J Am Coll Cardiol 2013;61:302-10. [Crossref] [PubMed]
- Paul TK, Bhatheja S, Panchal HB, et al. Outcomes of saphenous vein graft intervention with and without embolic protection device: A comprehensive review and meta-analysis. Circ Cardiovasc Interv 2017;10:e005538. [Crossref] [PubMed]
- Stone GW, Rogers C, Hermiller JRandomized Evaluation Investigators, et al. Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aortocoronary bypass grafts. Circulation 2003;108:548-53. [Crossref] [PubMed]
- Mauri L, Cox D, Hermiller J, et al. The PROXIMAL trial: Proximal protection during saphenous vein graft intervention using the Proxis Embolic Protection System: A randomized, prospective, multicenter clinical trial. J Am Coll Cardiol 2007;50:1442-9. [Crossref] [PubMed]
- Parasca CA, Head SJ, Milojevic MSYNTAX Investigators, et al. Incidence, characteristics, predictors, and outcomes of repeat revascularization after percutaneous coronary intervention and coronary artery bypass grafting: The SYNTAX trial at 5 years. JACC Cardiovasc Interv 2016;9:2493-507. [Crossref] [PubMed]
- Morrison DA, Sethi G, Sacks J, et al. Investigators of the Department of Veterans Affairs Cooperative Study #385. Angina with Extremely Serious Operative Mortality Evaluation. Percutaneous coronary intervention versus repeat bypass surgery for patients with medically refractory myocardial ischemia: AWESOME randomized trial and registry experience with post-CABG patients. J Am Coll Cardiol 2002;40:1951-4. [Crossref] [PubMed]
- Morrison DA, Sethi G, Sacks J, et al. Angina with Extremely Serious Operative Mortality Evaluation (AWESOME). Percutaneous coronary intervention versus coronary artery bypass graft surgery for patients with medically refractory myocardial ischemia and risk factors for adverse outcomes with bypass: A multicenter, randomized trial. Investigators of the Department of Veterans Affairs Cooperative Study #385, the Angina with Extremely Serious Operative Mortality Evaluation (AWESOME). J Am Coll Cardiol 2001;38:143-9. [Crossref] [PubMed]
- Harskamp RE, Beijk MA, Damman P, et al. Clinical outcome after surgical or percutaneous revascularization in coronary bypass graft failure. J Cardiovasc Med (Hagerstown) 2013;14:438-45. [Crossref] [PubMed]
- Kamdar AR, Meadows TA, Roselli EE, et al. Multidetector computed tomographic angiography in planning of re-operative cardiothoracic surgery. Ann Thorac Surg 2008;85:1239-45. [Crossref] [PubMed]
- Gold JP, Torres KE, Maldarelli W, et al. Improving outcomes in coronary surgery: the impact of echo-directed aortic cannulation and perioperative hemodynamic management in 500 patients. Ann Thorac Surg 2004;78:1579-85. [Crossref] [PubMed]
- Yau TM, Borger MA, Weisel RD, et al. The changing pattern of re-operative coronary surgery: trends in 1230 consecutive reoperations. J Thorac Cardiovasc Surg 2000;120:156-63. [Crossref] [PubMed]
- Roselli EE, Pettersson GB, Blackstone EH, et al. Adverse events during reoperative cardiac surgery: Frequency, characterization, and rescue. J Thorac Cardiovasc Surg 2008;135:316-23. [Crossref] [PubMed]
- Elami A, Laks H, Merin G. Technique for reoperative median sternotomy in the presence of a patent left internal mammary artery graft. J Card Surg 1994;9:123-7. [Crossref] [PubMed]
- Grunwald RP. A technique for direct vision sternal re-entry. Ann Thorac Surg 1985;40:521-2. [Crossref] [PubMed]
- Higami T, Kozawa S, Asada T, et al. Skeletonization and harvest of the internal thoracic artery with an ultrasonic scalpel. Ann Thorac Surg 2000;70:307-8. [Crossref] [PubMed]
- Navia D, Cosgrove DM 3rd, Lytle BW, et al. Is the internal thoracic artery the conduit of choice to replace a stenotic vein graft? Ann Thorac Surg 1994;57:40-3; discussion 43-4. [Crossref] [PubMed]
- Wendler O, Tscholl D, Huang Q, et al. Free flow capacity of skeletonized versus pedicled internal thoracic artery grafts in coronary artery bypass grafts. Eur J Cardiothorac Surg 1999;15:247-50. [Crossref] [PubMed]
- Dohi M, Doi K, Okawa Y, et al. Upgrading redo coronary artery bypass graft by recycling in situ arterial graft. Ann Thorac Surg 2014;98:311-4. [Crossref] [PubMed]
- Min HK, Lee YT, Kim WS, et al. Complete revascularization using a patent left internal thoracic artery and variable arterial grafts in multivessel coronary reoperation. Heart Surg Forum 2009;12:E244-9. [Crossref] [PubMed]
- Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularization on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001;358:870-5. [Crossref] [PubMed]
- Svensson LG, Mumtaz MA, Blackstone EH, et al. Does use of a right internal thoracic artery increase deep wound infection and risk after previous use of a left internal thoracic artery? J Thorac Cardiovasc Surg 2006;131:609-13. [Crossref] [PubMed]
- Glineur D, Noirhomme P, Poncelet A, et al. Gatroepiploic artery minimally invasive grafting in reoperative patients with patent mammaries. Ann Thorac Surg 2005;79:1606-9. [Crossref] [PubMed]
- Tavilla G, Bruggemans EF. Avoiding sternotomy in repeat coronary artery bypass grafting: feasibility, safety, and mid-term outcome of the transabdominal off-pump technique using the right gastroepiploic artery. J Thorac Cardiovasc Surg 2012;144:124-9. [Crossref] [PubMed]
- Maltais S, Widmer RJ, Bell MR, et al. Reoperation for coronary artery bypass grafting surgery: outcomes and considerations for expanding interventional procedures. Ann Thorac Surg 2017;103:1886-92. [Crossref] [PubMed]
- Ueki C, Miyata H, Motomura N, et al. Off-pump versus on-pump coronary artery bypass grafting in patients with left ventricular dysfunction. J Thorac Cardiovasc Surg 2016;151:1092-8. [Crossref] [PubMed]
- Sepehripour AH, Saso S, Harling L, et al. Does off-pump coronary revascularization reduce mortality in re-operative coronary artery surgery? A meta-analysis of observational studies. Perfusion 2013;28:340-9. [Crossref] [PubMed]
- Taggart DP, Aratari C, Wong P, et al. Applicability of intermittent global ischemia for repeat coronary artery operations. J Thorac Cardiovasc Surg 1996;112:501-7. Erratum in: J Thorac Cardiovasc Surg 1996 Dec;112(6):1484 Atari C [corrected to Aratari C]. [Crossref] [PubMed]
- Tugtekin SM, Alexiou K, Kappert U, et al. Coronary reoperation with and without cardiopulmonary bypass. Clin Res Cardiol 2006;95:93-8. [Crossref] [PubMed]
- Morris CD, Puskas JD, Pusca SV, et al. Outcomes after off-pump reoperative coronary artery bypass grafting. Innovations (Phila) 2007;2:29-32. [Crossref] [PubMed]
- Vohra HA, Bahrami T, Farid S, et al. Propensity score analysis of early and late outcome after redo off-pump and on-pump coronary artery bypass grafting. Eur J Cardiothorac Surg 2008;33:209-14. [Crossref] [PubMed]
- Kara I, Cakalagaoglu C, Ay Y, et al. Reoperative coronary artery bypass surgery: the role of on-pump and off-pump techniques on factors affecting hospital mortality and morbidity. Ann Thorac Cardiovasc Surg 2013;19:435-40. [Crossref] [PubMed]
- Puskas JD, Thourani VH, Kilgo P, et al. Off-pump coronary artery bypass disproportionately benefit high-risk patients. Ann Thorac Surg 2009;88:1142-7. [Crossref] [PubMed]
- Sedrakyan A, Wu AW, Parashar A, et al. Off-pump surgery is associated with reduced occurrence of stroke and other morbidity as compared with traditional coronary artery bypass grafting: a meta-analysis of systematically reviewed trials. Stroke 2006;37:2759-69. [Crossref] [PubMed]
- Doi K, Yaku H. Importance of cerebral artery risk evaluation before off-pump coronary artery bypass grafting to avoid perioperative stroke. Eur J Cardiothorac Surg 2010;38:568-72. [Crossref] [PubMed]
- Dohi M, Miyata H, Doi K, et al. The off-pump technique in redo coronary artery bypass grafting reduces mortality and major morbidities: propensity score analysis of data from the Japan Cardiovascular Surgery Database. Eur J Cardiothorac Surg 2015;47:299-307; discussion 307-8. [Crossref] [PubMed]
- Azoury FM, Gillinov M, Lytle BW, et al. Off-pump reoperative coronary artery bypass grafting by thoracotomy: patient selection and operative technique. Ann Thorac Surg 2001;71:1959-63. [Crossref] [PubMed]
- Shahian DM, O’Brien SM, Filardo G. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 1—coronary artery bypass grafting surgery. Ann Thorac Surg 2009;88:S2-22. [Crossref] [PubMed]
- Lytle BW, McElroy D, McCarthy P, et al. Influence of arterial coronary bypass grafts on the mortality in coronary reoperations. J Thorac Cardiovasc Surg 1994;107:675-82. [Crossref] [PubMed]
- Di Mauro M, Iaco AL, Contini M, et al. Reoperative coronary artery bypass grafting: analysis of early and late outcomes. Ann Thorac Surg 2005;79:81-7. [Crossref] [PubMed]
Cite this article as: Sajja LR. A narrative review of redo coronary artery bypass grafting. AME Med J 2021;6:20.


