
Management of locally advanced renal cell carcinoma
Background
In the United States, there are approximately 74,000 new cases and almost 15,000 deaths from renal parenchymal and pelvis cancer each year with the majority of these cases attributed to renal cell carcinoma (RCC) (1). RCC is more common in men than women and typically presents in older patients with an average age of 64 years (1). At presentation, the incidence of clinically localized RCC accounts for 65% of cases, while 16% had regional spread and 16% had distant metastatic disease. Extension into the renal vein or inferior vena cava (IVC) has been reported in 4–10% of cases. The 5-year survival rate for kidney cancer, between 2005 and 2015, was just over 75%. When separated by stage at diagnosis, the 5-year survival rate was 92% for localized, 68% for regional, and 12% for distant spread (2).
The triad of hematuria, flank pain, and flank mass are historically associated with renal cell carcinoma, but fewer than 10% of cases present with all three symptoms and even in the pre-CT era over 40% presented with none of the three (3). Currently, a large number of these cancers are detected incidentally due to increasing use of cross sectional abdominal imaging. In a review of 3,001 patients without symptoms imaged by computed tomography colonography, 14% of patients harbored a renal mass greater than 1 cm in size (4), and another review of patients surgically treated for renal masses showed that 15% presented without symptoms, and were diagnosed via findings on incidental scans. Those patients who were incidentally diagnosed on cross-sectional imaging had less advanced lesions and a longer 5-year specific survival rate (5). However, nearly 25% of contemporary patients are still diagnosed with advanced disease, which includes either distant or nodal metastases (NM) (6).
Renal cell carcinoma is the most common cause of renal cancer but it is a non-specific term utilized for a number of different cancers arising in the kidney. This classification includes the three most common histologic types, clear cell (ccRCC), papillary (pRCC), and chromophobe (chRCC) as well as other less-common histologic types that arise from the renal parenchyma and collecting system (7). While these cancers are commonly lumped into a group and treated similarly, they in fact arise from distinct cell types within the kidney and as a result have different behavior and progression patterns. Most of these cancers have a genetic syndrome associated with non-sporadic cancer etiology. Clear cell RCC is associated with von Hippel Lindau disease, pRCC is subdivided into type 1 and type 2 which are associated with germline mutations in MET, Hereditary Leiomyomatosis, and fumarate hydratase mutations, and chRCC is associated with Birt-Hogg-Dube disease (8). These cancers are typically characterized histologically, but recent studies have shown genetic alterations in addition to those seen in their genetic syndromes (9) highlighting the possibility of tailored treatments based on patient and tumor-specific genotype.
Cancer staging considers size of the original tumor as well as the extent of that tumor’s local invasion and distant spread. At this time, the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging is the most commonly used and universally accepted staging system for cancer (10). The TNM system defines locally advanced RCC as having any of the following characteristics: extending into major veins, invading the adrenal gland, extending into the peri-renal or peri-pelvic fat, or invading beyond the Gerota fascia (11). In addition to TNM staging, recurrence risk scoring is also commonly used in RCC following surgical intervention. The two commonly used systems are the SSIGN or Leibovich score which uses the tumor stage, size, grade, and level of necrosis (12) as well as the UISS/UCLA which uses stage, grade, and ECOG performance status (13). These two scoring systems classify the risk of recurrence following surgical resection and classify patients with ccRCC into low, intermediate, and high-risk groups for recurrence and have both been independently validated (14,15).
Renal cell carcinoma, and more specifically the most common subtype clear cell, does not follow the same progression as many other cancers. It is unique in that it is relatively resistant to radiotherapy and does not respond to classical chemotherapy (16). In addition, previous studies have shown hematogenous spread is common in ccRCC while lymphatic involvement less commonly precedes metastatic disease (17). For these reasons, management of locally advanced renal cell carcinoma presents unique difficulties and its management is fraught with several controversial topics. Uncertainties surround the role of lymphadenectomy in treatment and work continues to best define the role of biopsy prior to surgical intervention, especially in higher stage malignancy. Meanwhile, the role of performance status and metabolic predictors of outcome continue to evolve as the timing and sequencing of targeted agents, immunotherapy and surgery is better understood.
Despite the historical ineffectiveness of adjuvant therapies, new agents have shown great efficacy. Prior to the targeted therapy era interventions were cytokine-based immunotherapy in the form of INF-a and/or IL-2. First described in 2001 by Flanigan (18), these therapies showed only modest improvements in outcomes (ORR 5–31%) (19) and with them came a host of harsh side effects. With advances in the understanding of cellular mechanisms in ccRCC, specifically induction of hypoxia-induced factor (HIF) and consequent overexpression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) (20) a large number of new interventions targeting this pathway have emerged. Multiple agents against VEGF, PDGF, related receptors and inhibitors of the mammalian target of rapamycin (mTOR), as well as the MET and AXL tyrosine-protein kinase receptors, have been approved based on significant activity in RCC (20). Studies have been carried out with antiangiogenic drugs such as bevacizumab, sunitinib, sorafenib, and pazopanib in the adjuvant setting with promising results (20). Immunotherapy has also changed the role of role nephrectomy in locally advanced disease, and the sequence of treatment may differ due to recent studies that will be discussed later in this chapter (21,22). However, despite these significant advancements in medical management, the initial treatment of localized and locally advanced RCC remains surgical extirpation of disease.
Lymphadenectomy (LND)
LND has been shown to have staging, prognostic, and potentially therapeutic roles in prostate, bladder, penile, and testicular cancers (23-26). However, the role of LND in renal cancer remains very poorly defined. Two concepts form the rationale for a theoretical benefit to LND: in the non-metastatic setting, resection of all sites of disease which may include lymph nodes would be expected to be curative, while in the metastatic setting, cytoreduction of nodal disease might improve response to systemic therapy (27). To date, neither of these hypotheses have been adequately tested.
A few retrospective evaluations (28-31) and only one randomized trial (32) have been conducted on RCC in attempts to elucidate the value of LND. Treatment bias likely contributes greatly to the distortion of these data in the same fashion as for any oligometastatic disease: LND is only able to be contemplated in the setting of relatively limited disease and it is arguable that any benefit attributable to the procedure may be due to the selection of lower volume and less-aggressive disease (oligometastatic as opposed to widespread).
These retrospective studies suggested improved overall survival attributable to LND, but the subsequent Blom et al. randomized trial (EORTC 30881) found no benefit. A systematic review of a number of these retrospective studies and the RCT concluded that the evidence for LND was low due to the retrospective studies being underpowered and the variability of their results (33). One of the challenges in interpretation of EORTC 30881 has been that the study group was enriched with low risk patients, underpowering it to detect the benefit in higher risk patients (34). EORTC 30881 was a randomized, controlled trial that separated patients with clinically localized (N0M0), resectable renal mass consistent with RCC to radical nephrectomy alone or to radical nephrectomy plus a complete lymph node dissection. Primary endpoint was duration of survival. Target number of patients was 276 (138 in each arm), but due to rapid accrual this number was increased to approximately 700 to detect a 10% difference in an intention-to-treat analysis (30). Out of 346 patients who underwent lymph node dissection, only 14 (4%) were found to have lymph node metastases. This included 10 out of 51 patients with palpably enlarged lymph nodes and 4 out of 311 patients without palpable lymph nodes. Of the 365 patients who did not receive a formal LND, 33 had palpable lymph nodes which removed or biopsied, and 4 were found to have lymph node metastases. Overall, the event rate of lymph node metastasis in this predominantly low-risk study population was found to be low (30). A sub-analysis focusing only on cT3 tumors showed a 15% overall survival benefit at 5 years for LND recipients, although again the data was underpowered for this analysis (33). A more recent analysis of the value of LND on data from the ECOG 2805 adjuvant chemotherapy randomized trial by Ristau et al. also noted no improvement in OS in patients who underwent more aggressive surgical procedures, including LND (34). Most of the previously noted retrospective studies have been small and underpowered, but a more recent retrospective study from Gershman et al. had a much larger cohort of over 1,600 patients, and also revealed no improvement in OS or CSS, complementing the findings of Blom et al. and Ristau et al. In addition, the Gershman et al. study also attempted to identify a high-risk patient population for which LND was of benefit and were unable to do so (35). These findings suggest that at this time we have not clearly identified a patient group in which LND has shown a survival benefit. In the 2019 update of the EUA guidelines the authors did not make recommendations for the usage of LND (35,36). However, at this time the AUA guidelines recommend LND in the setting of clinically positive nodes either by imaging or palpable nodes during surgery (37). This recommendation can be attributed to the staging benefits of LND in patient selection for adjuvant treatment as well as persistent uncertainty in the setting of non-clear cell histology (38).
The absence of demonstrable benefit to LND in RCC may be the consequence of a few factors: LND is applied to the wrong patients, LND is being applied to the wrong nodes anatomically, or it truly lacks significant benefit. Lymphatic mapping studies have shown lympho-venous communications to the renal vein and IVC may exist, but even in the absence of this, the retroperitoneal lymphatic drainage is variable and not well understood (39). An aggregate review of 25 datasets of varying methodology found significant heterogeneity in templates; right sided LND typically included the renal hilar, paracaval, and precaval nodes, from the crus of the diaphragm to the aortic bifurcation. Left sided LND included the renal hilar, preaortic, and paraaortic nodes, from the crus of the diaphragm to the aortic bifurcation. This is demonstrated in Figure 1. Extended LND for both sides generally included the inter-aortocaval/retroaortic nodes and the affected side’s common iliac nodes (40).

While effectively all of the studies reported above document the number of LNs removed and the proportion of positive LNs at the time of surgery, very few described the specific anatomic location (and number) of positive nodes. Subsequent data has demonstrated great variance in the location of positive nodes, underscoring the challenges of assessing LND benefit (40,41). One study specifically designed to evaluate lymphatic drainage from RCC via sequential lymphoscintigraphy and sentinel node biopsy showed right-sided tumors predominantly drained into inter-aortocaval and retrocaval sentinel nodes and left sided tumors predominantly drained into para-aortic LNs (42). While these studies affirm the most common sites of nodal spread from RCC, they also highlight the immense variability of lymphatic drainage from the kidney. While a templated approach to LND may resect the most common nodes affected, the use of SPECT/CT sequential lymphoscintigraphy for a more specific approach may prove to be an effective tool to discerning an effective LND resection template.
LND is not without morbidity. Several studies assessing the value of LND have included peri-operative morbidity with complications occurring in 17% to 26% of cases (43), however several of these studies failed to find increased rate of peri-operative morbidity (34,35). These findings suggest that that LND probably carries an acceptable risk in patients during renal surgery performed by experienced retroperitoneal surgeons (34).
The possibility remains that even with technical and anatomic issues managed, LND simply may not add benefit. Early cadaver studies found that hematogenous spread is common and suggested lymphatic involvement almost never precedes metastatic disease (17). Mapping studies have shown direct lympho-venous communications to the renal vein and IVC, and some renal lymphatics have been shown to drain directly into the thoracic duct, facilitating distant spread (39). More recent studies have also found that lymph node involvement in clinically nonmetastatic RCC patients is less than 5% (44-46) and the incidence of nodal involvement in cT3 disease in the EORTC 30881 trial was only 6.3% (32). In addition, distant metastases are present in approximately 58–67% of patients with node positive disease (47,48).
RCC is a heterogenous group of cancers and non-clear cell histologies likely behave quite differently. A review assessing the survival outcomes in 1377 metastatic RCC patients with lymph node metastases separated ccRCC from non-ccRCC and found that non-ccRCC was more likely to have positive lymph nodes (67.3% vs. 54.4%) and were more likely to have isolated subdiaphragmatic lymph node metastases (34.0% vs. 16.9%) (49). These findings suggest that non-ccRCC may be a better candidate for lymphadenectomy; however, studies on the value of LND in non-ccRCC are lacking and further evaluation is needed to better delineate the differences between cc and non-ccRCC.
Evolving role of cytoreductive nephrectomy
While the majority of RCC is caught prior to metastasis, 32% of cases are metastatic upon presentation (2). Since the early 2000’s, the era of cytokine based chemotherapy, cytoreductive nephrectomy had been the standard of care for these patients due to two large case-controlled randomized trials indicating prolonged survival with upfront nephrectomy (18,50). A combined analysis of both studies demonstrated a significant median overall survival (OS) improvement of 5.8 months with CN and interferon compared with interferon alone (51). New understanding of the molecular mechanisms underlying renal cancer (52) has brought us inhibitors of vascular endothelial growth factor (VEGF) signaling. Adding the recent advent of immunotherapy agents, two randomized trials utilizing sunitinib in conjunction with cytoreductive nephrectomy in the setting of mRCC have been conducted. The first trial, Immediate Surgery or Surgery After Sunitinib Malate in Treating Patients With Metastatic Kidney Cancer (SURTIME), was a phase 3 RCT of immediate vs. deferred CN in patients with mRCC receiving sunitinib (21). The trial failed to achieve its objectives because of poor accrual. Of those patients that the study was able to evaluate, higher OS was observed in the group that underwent deferred CN, but the study was underpowered to draw any definitive conclusions (53). The second trial, the Clinical Trial to Assess the Importance of Nephrectomy (CARMENA) was a phase 3 noninferiority trial in intermediate- or poor-risk patients with mRCC randomized to CN followed by sunitinib vs. sunitinib alone. This trial also failed to meet its accrual goals but was able to demonstrate noninferiority for the non-CN experimental arm. Sunitinib-alone was favored compared to CN followed by sunitinib (OS 18.4 vs. 13.9 months), but this finding failed to reach statistically significance (22). To date, there have been no trials to inform the ideal treatment for good-risk mRCC. Furthermore, the study population of the CARMENA trial had significant heterogeneity. Patients with a low burden of metastatic pulmonary disease, with the majority of disease in the kidney, might still benefit from CN (54). Since that time, trials with newer therapies have shown Cabozantinib (55) as well as Nivolumab plus Ipilimumab (56) to be better first line agents in intermediate and poor risk mRCC patients.
Given that CN might still benefit a certain subgroup of patients with mRCC, it is important to note the morbidity and mortality associated with CN. Roussel et al. (2020) retrospectively analyzed data from 736 patients with mRCC undergoing CN and reported on the complications associated with the procedure. They found that 29.5% of patients had complications of any grade while 6.1% suffered a high-grade complication (Clavien-Dindo classification 3–5), and 1.4% died. Both low and high-grade complications were positively associated with estimated blood loss (EBL), suggesting that the complexity of surgery is associated with complications. Surgical volume was associated with significantly decreased odds of high-grade complications, suggesting that centralization of complex surgery for CN might decrease this morbidity (57).
In addition to these studies altering the sequence of multimodal therapy in some patients, they also suggest a value of identifying “responder” patient-tumor phenotypes and perhaps avoidance of non-beneficial CN. Previous retrospective studies of deferred CN reported that tumor shrinkage and reduction of neovascularization may facilitate resection (58,59) but have also raised concern of increased surgery-related adverse events following VEGF-targeted therapy (60,61). Review of the data produced by SURTIME showed that patients undergoing delayed nephrectomy had similar adverse event incidence to those undergoing initial nephrectomy 53% and 52% respectively (62). These findings have been echoed in the current treatment guidelines (63) although the benefit of CN in “responders” remains to be found.
In metastatic disease, surgical and targeted radiotherapeutic treatment of sites of metastasis is of unclear benefit. The literature has benefitted from systematic review of the available data on multiple modalities of treatment of site-specific metastasis, and while a benefit was suggested the data suffered from significant biases, are of entirely retrospective nature and in general represent the aggregation of many variable and non-controlled small series (64).
The role of CN is evolving, and the next areas of research should focus on identifying the clinical and pathologic features that can better select patients for the sequence of multimodal therapy.
Assessment of surgical suitability
Multiple approaches have been developed to assess patient readiness for major surgery. The concept of frailty has emerged over the past decade and has been found to be a significant predictor of surgical morbidity and mortality (65). The challenge in aggressive malignancy is the competing factors of disease progression and fatality, cast against this backdrop of often-advanced age and poor health. In general, 10–20% of the population over the age of 65 meets a definition of frailty, and as primary risks for RCC include obesity and smoking it is likely higher in those individuals (66,67). While there is no consensus definition of frailty, assessment has a role and can particularly factor into discussions where up-front chemotherapy or immunotherapy is being considered, as well as appropriate discussion of surgical risk.
The Fried frailty index is a validated 5-point measure that encompasses weight loss over 10 lbs, weakness of grip, subjective exhaustion, slow walking speed and low baseline activity; in general it can be administered in under 15 minutes (68). The so-called FRAIL scale consists of 5 direct questions that can be documented in under 5 minutes that includes the following: patient reports fatigue, patient cannot climb one flight of stairs, patient cannot walk one block, patient has greater than five illnesses, patient has lost over 5% of body weight (69). Given the time limitations and complex data management required to perform preoperative clearance in the elderly and comorbid patient, the most rapid assessments are the most likely to be used. Results should form the basis for a more informed discussion of surgical risk with the patient and family, as well as tumor board and high-risk committee meetings when appropriate.
Renal cell prognostic models
Two commonly used metric-based assessments of RCC prognosis are the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) and the somewhat older Motzer/Memorial Sloan Kettering criteria (70,71). These may be inappropriately used as assessments of surgical suitability; in fact, they are prognostic instruments for survival after treatment with targeted agents in the setting of metastatic disease. Specifically, they do not asses likelihood or significance of benefit from CN in the setting of locally advanced or metastatic disease. Neither do they integrate all factors that are comprise surgical frailty, although there is some overlap in the factors. The IMDC model’s negative predictors include low Karnofsky Performance Status (KPS) as well as time from diagnosis to first targeted therapy of over a year, low serum hemoglobin, high neutrophil and platelet counts, and elevated serum calcium levels. Patients possessing 3 or more of these factors fall into the poor-risk group, and in the initial report were found to have a median survival of 5.4 months, compared to intermediate risk patients with 1–2 risk factors (median survival 16.6 months) and good risk who enjoyed a median survival of 35.3 months. Interestingly, this model is predictive in non-clear cell histologies as well, and has also been shown to predict survival after second line treatment as well as initial therapy (72). Low serum sodium has been shown to predict poor outcomes in those treated with targeted therapy, although not necessarily immunotherapy (73).
Renal mass biopsy
Kidney cancer spans a spectrum from benign to aggressive disease and the malignant potential of a renal mass dictates clinical care. Size alone is a strong predictor of malignancy with one series of 2,770 patients who underwent resection of a solitary solid renal mass reporting that 46% of tumors less than 1 cm were benign, but only 6% of tumors greater than 7 cm were benign (74). In general, multiphasic cross-sectional imaging is the best modality to characterize renal masses and can help differentiate malignancy from common benign masses. CT and MRI assess for locally advanced features and intra-abdominal metastases (75). Given the fact that a large portion of renal masses are benign, renal mass biopsy can help direct care for these patients. Larger masses can also benefit from biopsy, because the histologic subtype can direct treatment. This can also be helpful in patients who are can’t tolerate or are reticent to pursue upfront surgery.
Percutaneous biopsy of renal masses can reveal the histology of indeterminate lesions. As such it may prove a valuable tool for patients considering resection, ablation or surveillance. In addition to masses in the kidney, biopsy can also identify distant metastatic lesions and quadrant biopsy of metastatic renal masses may inform chemotherapeutic planning (76). Historical concerns of limited diagnostic accuracy, risk of malignant seeding of the biopsy tract, and bleeding risk have been largely allayed. Several retrospective assessments of these questions have endorsed the safety of biopsy: a meta-analysis of 5,228 patients showed spontaneously resolving subcapsular/perinephric hematomas in 4.3% of cases in a pooled analysis, but clinically significant bleeding was unusual (0–1.4%/0.7% in the pooled analysis) and generally self-limited (77).
Percutaneous sampling can be performed under local anesthesia with needle core biopsy and/or fine needle aspiration (FNA) with either US or CT guidance. Both modalities provide a similar diagnostic yield (78). A coaxial technique allowing multiple biopsies through a coaxial cannula should always be used and eighteen-gauge needles are ideal for core biopsies, as they result in low morbidity and provide sufficient tissue for diagnosis (79). The same meta-analysis showing the safety of biopsy also showed that sensitivity and specificity of diagnostic core biopsies for the diagnosis of malignancy are 99.1% and 99.7%, respectively (77). However, 8% of samples in the meta-analysis were non-diagnostic and other studies have shown non-diagnostic rates as high as 20% (78). In the event of non-diagnostic sampling in a patient that has a mass on imaging concerning for malignancy, re-biopsy should be considered if the results would change management. Repeat biopsies have been reported to be diagnostic in a high proportion of cases with the lowest repeat diagnostic rates being over 80% (78). Larger masses have been shown to have significant heterogeneity, which can lead to sampling error with single core biopsy (80,81). This can be improved by the four-quadrant biopsy technique, in which four separate, solid, enhancing areas of the tumor are each biopsied. In one series which evaluated quadrant biopsy (76) versus standard biopsy (46) in 116 patients, standard biopsy was nondiagnostic in 5/46 biopsies, while multiquadrant biopsy was nondiagnostic in 0/76 biopsies (P=0.007) (76). This technique also had higher sensitivity for identifying sarcomatoid features, thus allowing the avoidance of unhelpful therapy.
Renal mass biopsy can be a valuable tool for evaluation of small renal masses as well as metastatic renal cancer to guide management. Patients should, however, be counseled that the biopsy is nondiagnostic in approximately 14% of cases. This can be improved with repeat biopsy. Biopsy should be employed only when it has the potential to change management. Biopsy should not be used in cases of young, healthy patients who cannot accept the uncertainties of biopsy, or in frail, older patients who will be managed conservatively regardless of findings. The use of biopsy is safe with minimal complication rates and has a high sensitivity and specificity for histologic typing when samples are diagnostic. Non-diagnostic rates are relatively low, and repeat biopsy can often provide diagnostic samples; of note, biopsy generally does not provide accurate grading of malignant lesions.
Surgical indications and preoperative considerations
Locally advanced disease may present a variety of surgical challenges, from invasion of adjacent structures to the increased risk of significant blood loss. Operating on large masses, especially via laparoscopic technique, can be challenging due to a lack of free space in which to maneuver as well as distorted anatomic relationships.
Preoperative renal artery embolization (PRAE) has been studied primarily in the setting of open surgery, with mixed results. A more recent RCT of PRAE vs. no-PRAE for complex tumors and including roughly half of patients undergoing laparoscopic surgery showed a decrease of 150 cc in blood loss in the group that underwent preoperative embolization, with no significant difference in surgical time, complication rate or length of stay (82). Another study reviewed cases of radical nephrectomy with IVC thrombectomy, and did not find any benefit to PRAE (83). Both of these studies were largely negative and found little, if any, benefit from PRAE. There were no complications specific to PRAE in either of these studies, though adjacent organ damage, coil migration, and PRAE-related death are potential complications from embolization procedures and may occur with higher volume.
Renal cell carcinoma, especially the more aggressive types, can invade directly into adjacent organs including liver, spleen, bowel and diaphragm. In the absence of clear metastasis and good surgical suitability, wide resection of all involved structures is warranted if within the abilities of the treating hospital and the patient is appropriately fit for surgery. The frequency of these scenarios is low enough that there are few validated strategies, and we recommend assessing each patient in a multispecialty setting. En-bloc resection of all involved structures appears desirable, but data has not progressed beyond isolated reports.
In the current era of effective targeted and immune therapies, neoadjuvant downstaging with subsequent surgery has been contemplated. This approach was first reported in 9 patients treated with sorafenib or sunitinib in 2008 (84). It suggested promising results both from notable simplification of subsequent surgery in some cases and a good side effect profile. A subsequent study of 19 patients that were felt to be unsuitable for surgery found a 24% shrinkage of primary tumor in 8, but 9 patients progressed while on therapy. Four of the original 19 (21%) ultimately did become resectable and underwent surgery, while 5 died of progressive cancer inside 6 months (60). Surgical complications did not appear higher in these series. Multiple experiences have been reported since and it appears that neoadjuvant therapy, generally 2 cycles of sunitinib, will result in 25–30% of unresectable tumors becoming resectable while nearly that amount will experience cancer progression on therapy. More than 2 cycles of sunitinib is associated with an undesirable increase in operative adhesions. There is no data currently available suggesting that neoadjuvant therapy and completion of nephrectomy has an impact, either positive or negative, on cancer-specific outcomes (85).
Surgical management of locally advanced kidney cancer
While the management of complex and invasive kidney cancer may be accomplished via either open or laparoscopic techniques, large masses provide additional complications and complexities that must be within the capacity of the surgical team and institution to manage. The routine performance of a bowel prep is not necessary unless colonic resection is felt likely; however we will administer oral magnesium citrate in patients that may be experiencing significant constipation, such as from pain medication, in order to decompress the colon. Aggressive DVT prophylaxis via both mechanical and pharmacologic means is important.
Open surgical options
Classic flank subcostal and supracostal incisions are, in general, poorly suited to the extirpation of large or complex masses, and less favorable to lymphadenectomy if indicated. Wide exposure and clear visualization are key, and usage of retractors such as the Omni-tract or Bookwalter is important to allow unhindered access to key areas. Anterior and transperitoneal incisions are likely best suited to extensive dissections.
Radical nephrectomy, as classically described, includes removal of all the contents of Gerota’s fascia. Routine adrenalectomy is not generally necessary unless there is suspicion of direct invasion or metastatic suspicion on imaging. That said, large or invasive masses that abut the adrenal region but without clear evidence of invasion are likely most safely treated with aggressive radical nephrectomy including adrenalectomy.
Best selection of incision should be based on surgeon familiarity and patient specifics.
Midline incision: This approach has the benefit of reproducibility, lack of positioning challenges and related complications such as rhabdomyolysis which are more common in flank positions. It affords excellent access to midline vascular structures. Additionally, when operating on a difficult right sided mass, this position supports arterial control in the interaortocaval space, which can simplify the direct dissection of the right renal hilum. Access to upper poles may be difficult depending on habitus, and vigorous retraction is often necessary. Patients with higher BMI or large antero-posterior dimension may be more challenging, but the midline incision reserves the option to be extended inferiorly or cranially into the chest when necessary. Closure is relatively direct and no significant muscle groups are divided, which may contribute to rapid recovery.
Chevron incision: Essentially an expansion of the anterior subcostal incision, this incision allows excellent exposure of all midline vascular structures and facilitates liver mobilization which is necessary for extended vena caval exposure and tumor thrombectomy. The incision is made beginning roughly at the tip of the 11th rib on the affected side and mirrors the costal margin roughly two fingers below. The extent of the incision on the contralateral side may extend fully to the tip of the opposite 11th rib or may be minimized if unnecessary.
Thoracoabdominal Incision: This incision is significantly morbid but provides the experienced surgeon outstanding access to upper pole tumors and especially liver or splenic involvement as well as access to the thoracic vena cava. The patient is placed in mild flank or semi-oblique position but with the pelvis essentially flat or nearly flat on the gel-padded bed. Incision is carried through multiple muscle groups, often resulting in significant postoperative pain and frequently the need for a chest tube. Preoperative epidural placement should be considered, and adequate pulmonary reserves are important on the patient’s part.
Development of the nephrectomy
In large and invasive carcinoma, the surgeon may discover the usual tissue planes are poorly defined, inflamed and adhesive, and often occupied by parasitic vessels. The anterior pararenal space especially is prone to challenges as a consequence of the adjacent mesocolon, and care must be taken not to devitalize this structure. Progression postero-medially on right sided tumors will bring one to the duodenum, which usually requires wide and athermal medialization via the Kocher maneuver, and the inferior vena cava. Injury to either of these structures can be catastrophic. Splenic injury on the left side is generally a result of vigorous traction that tears the capsule of the organ via the ligamentous attachments of the kidney and colon; all attempts should be made to release this structure early in the dissection to mitigate the risk of injury, bleeding and unnecessary splenectomy. Generally, we prefer to defer vascular control of the kidney until the later portions of the case during open surgery, or at least until enough mobility of the kidney is present such that hilar challenges can be addressed with relative ease. Damage to unrecognized lumbar or parasitic vessels can result in significant bleeding and is best managed in a setting where the kidney is mostly free or easy to remove.
Operations on large masses carry increased risk of disorientation and a surgeon may follow the contour of a particularly large mass to reach the wrong aspect of the great vessels; this risk is even greater during laparoscopic surgery. This can result in misidentification of renal artery and damage to the superior mesenteric artery or contralateral renal artery. Surgical planning and attention to non-hilar dissection during the early development of the case may be key.
Laparoscopic and robot-assisted radical nephrectomy
Minimally invasive surgery of the kidney is ever-more common and has been shown to be perfectly useful on complex masses, including caval thrombectomy in the hands of the highly experienced. The risks of disorientation are higher with this approach, due to the up-close perspective of the camera operating in a smaller space. Liberal use of intraoperative ultrasound may help to diminish these risks. Patients must be fit enough and possess adequate pulmonary reserves to exchange the CO2 load of laparoscopy, and unnecessarily prolonged surgery in the flank position, especially on men or high BMI patients, should be avoided to minimize risk of rhabdomyolysis.
Adrenalectomy
Adrenalectomy is not a necessary part of radical nephrectomy, but in the presence of a large upper pole mass or any suspicion of invasion adrenalectomy can be necessary for oncologic purposes. Preoperative imaging should be assessed for the presence of a viable contralateral gland. Routine ipsilateral adrenalectomy has not been shown to provide better cancer control, and some studies suggest it may be associated with worse overall survival (86,87). Routine ipsilateral adrenalectomy also puts patients at risk for asynchronous adrenal metastasis in a solitary gland. Given these findings, ipsilateral adrenalectomy should only be performed in patients with radiographic or intraoperative evidence of adrenal involvement (86,87).
Caval thrombectomy
Involvement of the renal venous system occurs in 4–10% of cases of RCC (88,89). However, this does not necessarily portend a fatal prognosis: 40–70% of patients with venous involvement can be cured with aggressive surgical resection (90,91). The tumor thrombus can be staged using the following system: level 0 thrombi are confined to the renal vein, level I thrombi extend no more than adjacent to the ostium of the renal vein, level II thrombi extend to below the hepatic veins, level III extend intra-hepatically but below the diaphragm, and level IV thrombi extend superior to the diaphragm (92).
Tumor level is generally assessed by MRI, though CT can be adequate. Though patients with locally advanced disease can be cured surgically, higher level is associated with poorer outcomes. Two large multi-institutional cohort studies revealed this: Martinez-Salamanca et al. (2011) showed that the 5-year survival after radical nephrectomy with thrombectomy for tumor thrombi involving the renal vein only was 43.2%, involving the IVC below the diaphragm was 37%, and fell to 22% for thrombi above the diaphragm (91). Smaller studies show conflicting data (93). A multi-institutional cohort study in the US found that tumor thrombus level is a significant predictor of recurrence (94).
Surgical technique varies by thrombus level and becomes more involved with increasing level. Level IV and even level III thrombi can require vascular bypass. Level IV thrombi often require a combined abdominal and thoracic approach. Still, many patients with level IV thrombi can be cured surgically, making IVC thrombectomy an in important operation for these patients. This has been shown in multiple series (95-98).
In general, care should be taken to preserve at least 50% of the IVC lumen, but oncologic control is paramount, and the IVC must be resected if there is thrombus extension into the IVC wall. Repair may require prosthetic interposition grafts or patch repair. These techniques will be discussed later in the chapter. Some patients with a completely occluded IVC may have completely developed collaterals and do not require reconstruction (99,100).
Robot assisted laparoscopic radical nephrectomy with IVC thrombectomy is an emerging practice. Abaza (2011) described the first series of 5 patients with level I and level II thrombi (101). Other case series have described outcomes of patients with up to level IV thrombi with 1 year follow up. These studies have shown reasonable perioperative outcomes with one year follow up, but long term oncologic outcomes are not yet available and the broad adoption of this technique is yet unclear (102,103).
Patients with level I-IV thrombi have been shown to have acceptable rates of morbidity and mortality, and long term survival is possible in a large portion of patients (104). RCC with IVC involvement is a surgically managed disease. It provides a chance for cure for those patients without evidence of metastatic disease, and can prevent devastating complications such as pulmonary emboli, intractable edema, ascites, and cardiac dysfunction in patients with metastatic disease.
Surgical technique
Surgical technique (105,106) does vary by thrombus level, but generally begins with ligation of the arterial blood supply, and the kidney is carefully mobilized leaving the renal vein as the only attachment. Incision selection is as reviewed above, and the surgeon should strive to achieve wide and unimpaired exposure.
Level I
For level I thrombi, the colon is reflected, the duodenum is athermally Kocherized (if tumor is on the right side), and the anterior pararenal space is broadly developed cranially and caudally to expose the great vessels. The renal artery is ligated but generally not divided using silk ligature or clips when accessible. The kidney is carefully mobilized above and below the renal vein, ureter is divided, and IVC freed up superior and inferior to the renal vein, leaving the renal vein as the only attachment remaining. The IVC may be milked to the ostium of the renal vein, and a C-shaped Satinsky clamp is then placed around the ostium of the renal vein after ensuring the thrombus is entirely within the jaws of the clamp before closing (Figure 2). The renal ostium is then sharply incised and thrombus extracted in its entirety and wrapped in gauze. Though vascular control of the IVC and contralateral renal vein is usually unnecessary in level I thrombi, it can be useful if there is any doubt as to the level of the thrombus. The contralateral renal vein is isolated with a vessel loop, then the same is performed for the suprarenal and infrarenal IVC. The vessel loops can be passed through an 18F red rubber catheter to create Rummel tourniquets. Manually pinch the IVC closed as cranially as possible, then apply Rummel tourniquets in the following order: infrarenal IVC, contralateral renal vein then suprarenal IVC (this is displayed in Figure 3). The ostium of the renal vein is then clamped and incised to extract the thrombus as described above. The IVC is carefully inspected for residual thrombus, irrigated with heparinized saline solution, then closed using 4-0 Prolene. Before tying the knot, anesthesia should apply positive airway pressure, the infrarenal IVC is pinched closed and the Satinsky clamp is released allowing ~10 mL blood to discharge which flushes out any residual thrombus. The suture is then pulled tight and tied closed.


Level II
Level II caval thrombectomy follows a similar pattern as described above for level I thrombi but requires more extensive dissection often including ligation and division of lumbar veins. Midline and chevron incisions provide best access for left sided tumors as the ascending and descending colons must be mobilized to gain exposure to the IVC in addition to the affected kidney. For left sided tumors, ligate and divide the gonadal, adrenal, and lumbar branches of the renal vein as needed. As above for level I tumors, the kidney is carefully mobilized and ureter divided. If not already performed (in the case of left sided tumors), the ascending colon is mobilized along the white line of Toldt, Kocher maneuver performed and the right anterior pararenal space is developed to expose the great vessels. The IVC is dissected from the liver to its bifurcation, ligating the gonadal vein in the process. During this portion of the procedure, it can be beneficial to dissect the lymphatics off of the IVC. At this stage in the procedure, vascular control is attained in the following order (similar technique as described above for level I thrombi): ipsilateral renal artery is ligated, the infrarenal IVC is clamped, then contralateral renal vein followed by the suprarenal IVC (Figure 3). IV heparin can be given prior to clamping to prevent thrombosis. For left sided tumors, the right renal artery can be clamped to avoid renal engorgement which may occur due to the paucity of collateral venous circulation available to decompress the kidney on the that side.
Once vascular control is obtained, the renal vein ostium is excised sharply (Potts scissors), and extended along the anterior aspect of the IVC exposing the thrombus. Open lumbar veins should be ligated as needed at this stage. The tumor is carefully extracted and removed en bloc with the kidney. As above the IVC lumen is flushed with heparinized saline then inspected for signs of invasion with biopsy of suspicious areas. The defect is closed with 4-0 Prolene and flushed in the same fashion as above, ensuring that the IVC lumen is greater than 50% of its original size. The contralateral renal clamp followed by the suprarenal IVC clamps are released and lymphadenectomy may be performed.
Level III and IV
Level III thrombi can either be accessed via an abdominal or combined abdominal and thoracic approach. This decision must be made after the renal artery is ligated and the liver is mobilized to allow for assessment of the cranial extent of the IVC. The venous circulation from the liver is substantial. That being said, it is ideal if the IVC can be clamped below the hepatic veins. If the thrombus extends above the hepatic veins, which should be assessed by TEE, bypass will be required if the thrombus is partially occlusive. However, if the thrombus is completely occlusive, then adequate collaterals have usually been formed and these patients will tolerate clamping without bypass.
Exposure of the kidney is performed as per level I and II thrombi and renal artery is performed. A preoperative plan regarding assistance, if needed, from vascular or liver/transplant surgeons should be established. As the case proceeds, Rummel tourniquets are placed around the infrarenal IVC and contralateral renal vein. The ligamentum teres is divided to begin mobilization of the liver. The falciform ligament is divided superiorly to the bifurcation into the coronary ligament and the left triangular ligament. The superior, then inferior layers of the coronary ligament are divided, paying close attention to avoid injury of the IVC or liver. Next, the left triangular ligament is divided anteriorly, then the posterior portions are divided toward the IVC. The liver can now be rotated toward midline to allow for inspection of the IVC. Tumor thrombus can also extend into the right, middle and left hepatic veins causing Budd-Chiari syndrome. These exist in a plane between the posterior liver and anterior IVC and should be inspected if there is concern for involvement. This group of veins cannot be ligated, however, the lower accessory hepatic veins can be ligated. Ligation of lumbar veins between the IVC and posterior abdominal wall completes the mobilization of the IVC.
If the IVC must be clamped above the major hepatic veins, then the Pringle maneuver (clamping of the porta hepatis) must be performed to prevent major blood loss. Though the porta hepatis can be clamped up to one hour, it is recommended to limit clamping to 20 minutes to minimize risk of hepatic ischemia, portal vein thrombosis and splenic engorgement.
As stated above, if the IVC can be clamped below the hepatic veins, then it is usually safe to proceed without bypass. This is displayed in Figure 4. If suprahepatic IVC clamping is required, then a clamping trial will determine whether or not bypass will be required (if cardiac output drops more than 50% or if MAP drops more than 30%). One series describing 78 patients who underwent IVC thrombectomy found that 8 requiring suprahepatic control of the IVC were completed without the need for bypass (104).
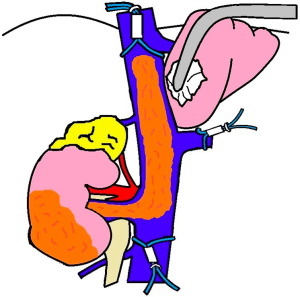
Level IV will often require a combined thoracic and abdominal approach. The clamping sequence is as follows: infrarenal IVC, contralateral renal vein, porta hepatis then suprahepatic IVC. The remainder of the procedure is for extraction of the mass and closure of the defect similar to level II thrombi.
Bypass considerations
In cases that have extensive IVC tumor or thrombus, adjunctive measures must be taken to preserve venous return from below the area of resection. The goal is to prevent hemodynamic instability from cross-clamping while preserving visualization in the absence of bleeding. Two of the potential methods to minimize these issues include venovenous bypass (VVB), and cardiopulmonary bypass (CPB).
VVB is a well-established technique developed for orthotopic liver transplantation, which has since been applied to radical nephrectomy with IVC thrombectomy. VVB necessitates a cannula below the level of resection or clamping, usually in the infrarenal IVC or femoral vein. This cannula will be connected to the inflow of the bypass machine to drain the venous return from the lower body. The outflow or return to the right side of the heart will arise from cannulation of either the internal jugular vein, axillary vein, subclavian vein, innominate vein, or if the chest cavity is open, the right atrium. If the portal vein is clamped, further drainage can be obtained from another cannula in the superior mesenteric vein. Of note, VVB does not require full heparinization or an oxygenator. Some reviews have suggested decreased operative time, length of hospitalization, intraoperative blood loss, and need for blood transfusions in VVB relative to CPB (97).
However, VVB is not an option with extensive level IV tumor thrombus that extends into or potentially embolizes into the pulmonary vasculature. Furthermore, VVB is not without risk, as there is an estimated complication incidence of 10% to 30% in VVB during liver transplantation (107), and as high as 30% in radical nephrectomy (97). However, complications in radical nephrectomy are also attributable to other procedural factors, and this number is further influenced by patient selection and surgical technique. These complications include bleeding, air, tumor or clot embolus to the lungs, hypothermia, bypass circuit and vessel thrombosis, vascular and nerve injury at the access site, and infection (107).
Full cardiopulmonary bypass may be necessary when the tumor extends either into the supradiaphragmatic intrapericardial IVC, or into the right atrium and beyond necessitating atriotomy (level IV tumor thrombi) (108). CPB is initiated with ascending aortic cannulation, a venous cannula placed into the SVC, and a second venous cannula placed either below the palpable thrombus in any vein in direct communication with the IVC. Depending on the extent of the tumor or thrombus, the extent of cooling can be tailored. In cases where the tumor can be controlled, isolation of the liver with a Pringle maneuver may allow resection of the tumor at normothermia while on bypass. Further extension of the tumor may require further cooling with cardiac arrest and aortic cross-clamping. The most extensive cases may require deep hypothermia and circulatory arrest to remove extension of embolus into the pulmonary arterial tree.
Important to consider is although cardiopulmonary bypass may provide temporary control of bleeding, bleeding following the bypass run can be significant due to coagulopathy intrinsic to the anticoagulation, hypothermia and the bypass circuit interaction to clotting factors. This will result in a higher rate of overall blood loss, and longer operative times (109).
Other potential complications of cardiopulmonary bypass can be minimized with preoperative and postoperative planning. Neurologic injury may occur as a result of aortic cannula placement, resulting in seeding of aortic plaque. If a patient has significant history of atherosclerotic disease, it may merit preoperative aortic imaging, or intraoperative transesophageal ultrasound. In spite of cardioplegia, subclinical myocardial injury can still occur. Immediate cardiac dysfunction may occur as a result of myocardial stunning, which is influenced by preoperative ventricular function, metabolic acidosis, and reperfusion injury. Optimizing electrolytes, temperature, and pH can help to reduce arrhythmia and improve stunning. Acute respiratory distress syndrome can be present due to the effects of CPB, and could be exacerbated by anesthesia-induced atelectasis. It is important to consider lung protective strategies in ventilation pre- and post-operatively. Lastly, acute kidney injury can occur as a result of inflammatory response and hypotension, especially in cases of prolonged bypass. Maintaining a high perfusion pressure is essential, especially given the need to preserve remaining kidney after tumor resection (109).
Overall, CPB during radical nephrectomy has a non-significant difference in total complication rate and five year mortality compared to VVB (97). Furthermore, a recent retrospective multi-institutional analysis found no significant difference in surgical complications, intraoperative and 30-day mortality, and hospital LOS in CPB versus non-CPB for level III/IV tumor thrombectomy. The decision on CPB usage should be made by a multispecialty team on the basis of surgical experience, perioperative imaging, and patient comorbidities (110).
As a whole, both of these methods pose significant perioperative and postoperative risks, however, it is far better to err on the side of caution, as initiation of bypass in a hemodynamically unstable patient significantly exacerbates these risks.
IVC reconstruction with patch or graft
As stated above, care should be taken to preserve at least 50% of the IVC lumen, but oncologic control is paramount, and the IVC must be resected if there is thrombus extension into the IVC wall. A double row of 4-0 Prolene sutures can be used for primary repair. If the IVC cannot be repaired primarily to preserve at least 50% of the IVC lumen and there is not adequate collateral flow, then a patch or graft can be used for reconstruction.
Material for patch reconstruction includes bovine and autologous pericardium, Dacron, PTFE and autologous saphenous vein. The patch can be either parachuted into place or sewn in by tacking the apices. Generally, a double-armed 5-0 Prolene on a BB needle is used, and care is taken to avoid inversion of the patch which can be thrombogenic. The inferior IVC clamp should be released prior to tying the last knot to allow for approximately 10cc blood to leak from the cavotomy, which clears the lumen of clot, air and debris prior to releasing the superior clamp.
Grafting may be required if the caval defect is too large for patching or is a circumferential resection of the IVC was performed. PTFE grafts are commonly, and are typically 16-20 mm in diameter. The superior anastomosis should be performed first. The superior portion of the graft is then clamped and the suprahepatic IVC clamp is released to test this anastomosis. The inferior anastomosis is then performed, trimming the graft to be as short as possible to avoid redundancy and promote laminar flow. Before tying the knot, the clamp on the graft should be released to allow 10cc blood to leak from the cavotomy. This is re-clamped and the infrarenal IVC clamp is released to allow an additional 10cc blood to leak from the cavotomy which clears the lumen of clot, air and debris. The final knot can then be tied and all clamps released. Retroperitoneal fat or omentum can be used to cover the graft, and hepatic ligaments reapproximated. Lifelong anticoagulation is indicated for these patients.
Acknowledgments
All figures were originally produced for this article by Zachary Pfeiffer, MD.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Simon P. Kim) for the series “Surgical Management of Genitourinary Malignancies” published in AME Medical Journal. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-20-79/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-79/coif). The series “Surgical Management of Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Vergho DC, Loeser A, Kocot A, et al. Tumor thrombus of inferior vena cava in patients with renal cell carcinoma - clinical and oncological outcome of 50 patients after surgery. BMC Res Notes 2012;5:5. [Crossref] [PubMed]
- Gibbons RP, Monte JE, Correa RJ Jr, et al. Manifestations of renal cell carcinoma. Urology 1976;8:201-6. [Crossref] [PubMed]
- O'Connor SD, Pickhardt PJ, Kim DH, et al. Incidental finding of renal masses at unenhanced CT: prevalence and analysis of features for guiding management. AJR Am J Roentgenol 2011;197:139-45. [Crossref] [PubMed]
- Tsui K-H, Shvarts O, Smith RB, et al. Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol 2000;163:426-30. [Crossref] [PubMed]
- Pantuck AJ, Zisman A. Belldegrun as. the changing natural history of renal cell carcinoma. J Urol 2001;166:1611-23. [Crossref] [PubMed]
- Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ 2014;349:g4797. [Crossref] [PubMed]
- Haas NB, Nathanson KL. Hereditary kidney cancer syndromes. Adv Chronic Kidney Dis 2014;21:81-90. [Crossref] [PubMed]
- Manley BJ, Hakimi AA. Molecular profiling of renal cell carcinoma: building a bridge toward clinical impact. Curr Opin Urol 2016;26:383-7. [Crossref] [PubMed]
- Amin MB, Edge SB. AJCC cancer staging manual. Springer; 2017.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. John Wiley & Sons; 2011.
- Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 2002;168:2395-400. [Crossref] [PubMed]
- Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol 2002;20:4559-66. [Crossref] [PubMed]
- Patard J-J, Kim HL, Lam JS, et al. Use of the University of California Los Angeles Integrated Staging System to Predict Survival in Renal Cell Carcinoma: An International Multicenter Study. J Clin Oncol 2004;22:3316-22. [Crossref] [PubMed]
- Zigeuner R, Hutterer G, Chromecki T, et al. External validation of the Mayo Clinic stage, size, grade, and necrosis (SSIGN) score for clear-cell renal cell carcinoma in a single European centre applying routine pathology. Eur Urol 2010;57:102-9. [Crossref] [PubMed]
- Porter JR. The Role of Lymphadenectomy for Renal Cell Carcinoma: Are we any Closer to an Answer? Eur Urol 2017;71:568-9. [Crossref] [PubMed]
- Johnsen JA, Hellsten S. Lymphatogenous Spread of Renal Cell Carcinoma: An Autopsy Study. J Urol 1997;157:450-3. [Crossref] [PubMed]
- Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy Followed by Interferon Alfa-2b Compared with Interferon Alfa-2b Alone for Metastatic Renal-Cell Cancer. N Engl J Med 2001;345:1655-9. [Crossref] [PubMed]
- Garcia JA, Rini BI. Recent progress in the management of advanced renal cell carcinoma. CA Cancer J Clin 2007;57:112-25. [Crossref] [PubMed]
- Barata PC, Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin 2017;67:507-24. [Crossref] [PubMed]
- Bex A, Mulders P, Jewett M, et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol 2019;5:164-70. [Crossref] [PubMed]
- Méjean A, Ravaud A, Thezenas S, et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N Engl J Med 2018;379:417-27. [Crossref] [PubMed]
- Joslyn SA, Konety BR. Impact of extent of lymphadenectomy on survival after radical prostatectomy for prostate cancer. Urology 2006;68:121-5. [Crossref] [PubMed]
- Kondo T, Tanabe K. Role of lymphadenectomy in the management of urothelial carcinoma of the bladder and the upper urinary tract. Int J Urol 2012;19:710-21. [Crossref] [PubMed]
- Hu B, Shah S, Shojaei S, et al. Retroperitoneal Lymph Node Dissection as First-Line Treatment of Node-Positive Seminoma. Clin Genitourin Cancer 2015;13:e265-9. [Crossref] [PubMed]
- O'Brien JS, Perera M, Manning T, et al. Penile Cancer: Contemporary Lymph Node Management. J Urol 2017;197:1387-95. [Crossref] [PubMed]
- Margulis V, Wood CG. The role of lymph node dissection in renal cell carcinoma: the pendulum swings back. Cancer J 2008;14:308-14. [Crossref] [PubMed]
- Sullivan LD, Westmore DD, McLoughlin MG. Surgical management of renal cell carcinoma at the Vancouver General Hospital: 20-year review. Can J Surg 1979;22:427-31. [PubMed]
- Peters PC, Brown GL. The role of lymphadenectomy in the management of renal cell carcinoma. Urol Clin North Am 1980;7:705-9. [PubMed]
- Siminovitch JP, Montie JE, Straffon RA. Lymphadenectomy in renal adenocarcinoma. J Urol 1982;127:1090-1. [Crossref] [PubMed]
- Herrlinger A, Schrott KM, Schott G, et al. What are the Benefits of Extended Dissection of the Regional Renal Lymph Nodes in the Therapy of Renal Cell Carcinoma? J Urol 1991;146:1224-7. [Crossref] [PubMed]
- Blom JHM, van Poppel H, Maréchal JM, et al. Radical Nephrectomy with and without Lymph-Node Dissection: Final Results of European Organization for Research and Treatment of Cancer (EORTC) Randomized Phase 3 Trial 30881. Eur Urol 2009;55:28-34. [Crossref] [PubMed]
- Bekema HJ, MacLennan S, Imamura M, et al. Systematic Review of Adrenalectomy and Lymph Node Dissection in Locally Advanced Renal Cell Carcinoma. Eur Urol 2013;64:799-810. [Crossref] [PubMed]
- Ristau BT, Manola J, Haas NB, et al. Retroperitoneal Lymphadenectomy for High Risk, Nonmetastatic Renal Cell Carcinoma: An Analysis of the ASSURE (ECOG-ACRIN 2805) Adjuvant Trial. J Urol 2018;199:53-9. [Crossref] [PubMed]
- Gershman B, Thompson RH, Boorjian SA, et al. Radical Nephrectomy with or without Lymph Node Dissection for High Risk Nonmetastatic Renal Cell Carcinoma: A Multi-Institutional Analysis. J Urol 2018;199:1143-8. [Crossref] [PubMed]
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol 2019;75:799-810. [Crossref] [PubMed]
- Campbell S, Uzzo RG, Allaf ME, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol 2017;198:520-9. [Crossref] [PubMed]
- Capogrosso P, Larcher A, Nini A, et al. The critical role of lymph node dissection in selecting high-risk nonmetastatic renal cancer candidates for adjuvant therapy after nephrectomy. Urol Oncol 2019;37:293.e25-293.e30. [Crossref] [PubMed]
- Karmali RJ, Suami H, Wood CG, et al. Lymphatic drainage in renal cell carcinoma: back to the basics. BJU Int 2014;114:806-17. [Crossref] [PubMed]
- Campi R, Sessa F, Di Maida F, et al. Templates of lymph node dissection for renal cell carcinoma: a systematic review of the literature. Front Surg 2018;5:76. [Crossref] [PubMed]
- Nini A, Larcher A, Cianflone F, et al. The effect of anatomical location of lymph node metastases on cancer specific survival in patients with clear cell renal cell carcinoma. Front Surg 2018;5:26. [Crossref] [PubMed]
- Kuusk T, Bruijn RD, Brouwer OR, et al. Lymphatic Drainage from Renal Tumors In-Vivo: A Prospective Sentinel Node Study Using SPECT/CT Imaging. J Urol 2018;199:1426-32. [Crossref] [PubMed]
- Bhindi B, Wallis CJD, Boorjian SA, et al. The role of lymph node dissection in the management of renal cell carcinoma: a systematic review and meta-analysis. BJU Int 2018;121:684-98. [Crossref] [PubMed]
- Hutterer GC, Patard J-J, Perrotte P, et al. Patients with renal cell carcinoma nodal metastases can be accurately identified: External validation of a new nomogram. Int J Cancer 2007;121:2556-61. [Crossref] [PubMed]
- Karakiewicz PI, Trinh Q-D, Bhojani N, et al. Renal Cell Carcinoma with Nodal Metastases in the Absence of Distant Metastatic Disease: Prognostic Indicators of Disease-Specific Survival. Eur Urol 2007;51:1616-24. [Crossref] [PubMed]
- Delacroix SE, Chapin BF, Chen JJ, et al. Can a Durable Disease-Free Survival be Achieved With Surgical Resection in Patients With Pathological Node Positive Renal Cell Carcinoma? J Urol 2011;186:1236-41. [Crossref] [PubMed]
- Giuliani L, Giberti C, Martorana G, et al. Radical Extensive Surgery for Renal Cell Carcinoma: Long-Term Results and Prognostic Factors. J Urol 1990;143:468-73. [Crossref] [PubMed]
- Pantuck AJ, Zisman A, Dorey F, et al. Renal Cell Carcinoma With Retroperitoneal Lymph Nodes: Role of Lymph Node Dissection. J Urol 2003;169:2076-83. [Crossref] [PubMed]
- Kroeger N, Pantuck AJ, Wells JC, et al. Characterizing the Impact of Lymph Node Metastases on the Survival Outcome for Metastatic Renal Cell Carcinoma Patients Treated with Targeted Therapies. Eur Urol 2015;68:506-15. [Crossref] [PubMed]
- Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001;358:966-70. [Crossref] [PubMed]
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071-6. [Crossref] [PubMed]
- Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N Engl J Med 2017;376:354-66. [Crossref] [PubMed]
- Lara PN Jr, Evans CP. Cytoreductive Nephrectomy in Metastatic Renal Cell Cancer: Not All That It's Cut Out to Be. JAMA Oncol 2019;5:171-2. [Crossref] [PubMed]
- Kuusk T, Szabados B, Liu WK, et al. Cytoreductive nephrectomy in the current treatment algorithm. Ther Adv Med Oncol 2019;11:1758835919879026. [Crossref] [PubMed]
- Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol 2017;35:591-7. [Crossref] [PubMed]
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378:1277-90. [Crossref] [PubMed]
- Roussel E, Campi R, Larcher A, et al. Rates and Predictors of Perioperative Complications in Cytoreductive Nephrectomy: Analysis of the Registry for Metastatic Renal Cell Carcinoma. Eur Urol Oncol 2020;3:523-9. [Crossref] [PubMed]
- Margulis V, Matin SF, Tannir N, et al. Surgical Morbidity Associated With Administration of Targeted Molecular Therapies Before Cytoreductive Nephrectomy or Resection of Locally Recurrent Renal Cell Carcinoma. J Urol 2008;180:94-8. [Crossref] [PubMed]
- Shuch B, Riggs SB, LaRochelle JC, et al. Neoadjuvant targeted therapy and advanced kidney cancer: observations and implications for a new treatment paradigm. BJU Int 2008;102:692-6. [Crossref] [PubMed]
- Thomas AA, Rini BI, Stephenson AJ, et al. Surgical Resection of Renal Cell Carcinoma After Targeted Therapy. J Urol 2009;182:881-6. [Crossref] [PubMed]
- Shaw GL, Hussain M, Nair R, et al. Performing Cytoreductive Nephrectomy following Targeted Sunitinib Therapy for Metastatic Renal Cell Carcinoma: A Surgical Perspective. Urol Int 2012;89:83-8. [Crossref] [PubMed]
- De Bruijn RE, Mulders P, Jewett MA, et al. Surgical Safety of Cytoreductive Nephrectomy Following Sunitinib: Results from the Multicentre, Randomised Controlled Trial of Immediate Versus Deferred Nephrectomy (SURTIME). Eur Urol 2019;76:437-40. [Crossref] [PubMed]
- Bex A, Albiges L, Ljungberg B, et al. Updated European Association of Urology Guidelines for Cytoreductive Nephrectomy in Patients with Synchronous Metastatic Clear-cell Renal Cell Carcinoma. Eur Urol 2018;74:805-9. [Crossref] [PubMed]
- Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol 2014;15:e549-61. [Crossref] [PubMed]
- Robinson TN, Walston JD, Brummel NE, et al. Frailty for Surgeons: Review of a National Institute on Aging Conference on Frailty for Specialists. J Am Coll Surg 2015;221:1083-92. [Crossref] [PubMed]
- Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487-92. [Crossref] [PubMed]
- Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010;58:681-7. [Crossref] [PubMed]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. [Crossref] [PubMed]
- Abellan van Kan G, Rolland YM, Morley JE, et al. Frailty: Toward a Clinical Definition. J Am Med Dir Assoc 2008;9:71-2. [Crossref] [PubMed]
- Heng DYC, Xie W, Regan MM, et al. Prognostic Factors for Overall Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor–Targeted Agents: Results From a Large, Multicenter Study. J Clin Oncol 2009;27:5794-9. [Crossref] [PubMed]
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and Prognostic Stratification of 670 Patients With Advanced Renal Cell Carcinoma. J Clin Oncol 1999;17:2530. [Crossref] [PubMed]
- Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015;16:293-300. [Crossref] [PubMed]
- Schutz FAB, Xie W, Donskov F, et al. The Impact of Low Serum Sodium on Treatment Outcome of Targeted Therapy in Metastatic Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Cancer Database Consortium. Eur Urol 2014;65:723-30. [Crossref] [PubMed]
- Frank I, Blute ML, Cheville JC, et al. Solid Renal Tumors: An Analysis of Pathological Features Related to Tumor Size. J Urol 2003;170:2217-20. [Crossref] [PubMed]
- Davenport MS, Hu EM, Smith AD, et al. Reporting standards for the imaging-based diagnosis of renal masses on CT and MRI: a national survey of academic abdominal radiologists and urologists. Abdom Radiol (NY) 2017;42:1229-40. [Crossref] [PubMed]
- Abel EJ, Heckman JE, Hinshaw L, et al. Multi-Quadrant Biopsy Technique Improves Diagnostic Ability in Large Heterogeneous Renal Masses. J Urol 2015;194:886-91. [Crossref] [PubMed]
- Marconi L, Dabestani S, Lam TB, et al. Systematic Review and Meta-analysis of Diagnostic Accuracy of Percutaneous Renal Tumour Biopsy. Eur Urol 2016;69:660-73. [Crossref] [PubMed]
- Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 2011;60:578-84. [Crossref] [PubMed]
- Veltri A, Garetto I, Tosetti I, et al. Diagnostic accuracy and clinical impact of imaging-guided needle biopsy of renal masses. Retrospective analysis on 150 cases. Eur Radiol 2011;21:393-401. [Crossref] [PubMed]
- Abel EJ, Culp SH, Matin SF, et al. Percutaneous biopsy of primary tumor in metastatic renal cell carcinoma to predict high risk pathological features: comparison with nephrectomy assessment. J Urol 2010;184:1877-81. [Crossref] [PubMed]
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med 2012;366:883-92. [Crossref] [PubMed]
- Cochetti G, Zingaro MD, Boni A, et al. Renal Artery Embolization Before Radical Nephrectomy for Complex Renal Tumour: Which are the True Advantages? Open Med (Wars) 2019;14:797-804. [Crossref] [PubMed]
- Subramanian VS, Stephenson AJ, Goldfarb DA, et al. Utility of preoperative renal artery embolization for management of renal tumors with inferior vena caval thrombi. Urology 2009;74:154-9. [Crossref] [PubMed]
- Amin C, Wallen E, Pruthi RS, et al. Preoperative tyrosine kinase inhibition as an adjunct to debulking nephrectomy. Urology 2008;72:864-8. [Crossref] [PubMed]
- Tomita Y. Treatment strategies for advanced renal cell carcinoma: A new paradigm for surgical treatment. Int J Urol 2016;23:13-21. [Crossref] [PubMed]
- Weight CJ, Kim SP, Lohse CM, et al. Routine Adrenalectomy in Patients with Locally Advanced Renal Cell Cancer Does Not Offer Oncologic Benefit and Places a Significant Portion of Patients at Risk for an Asynchronous Metastasis in a Solitary Adrenal Gland. Eur Urol 2011;60:458-64. [Crossref] [PubMed]
- Weight CJ, Mulders PF, Pantuck AJ, et al. The Role of Adrenalectomy in Renal Cancer. Eur Urol Focus 2016;1:251-7. [Crossref] [PubMed]
- Marshall FF. Renal cell carcinoma: surgical management of regional lymph nodes and inferior vena-caval tumor thrombus. Semin Surg Oncol 1988;4:129-32. [PubMed]
- Quencer KB, Friedman T, Sheth R, et al. Tumor thrombus: incidence, imaging, prognosis and treatment. Cardiovasc Diagn Ther 2017;7:S165-77. [Crossref] [PubMed]
- Blute ML, Leibovich BC, Lohse CM, et al. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int 2004;94:33-41. [Crossref] [PubMed]
- Martínez-Salamanca JI, Huang WC, Millan I, et al. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol 2011;59:120-7. [Crossref] [PubMed]
- Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol 1987;59:390-5. [Crossref] [PubMed]
- Ficarra V, Galfano A, Mancini M, et al. TNM staging system for renal-cell carcinoma: current status and future perspectives. Lancet Oncol 2007;8:554-8. [Crossref] [PubMed]
- Abel EJ, Masterson TA, Karam JA, et al. Predictive Nomogram for Recurrence following Surgery for Nonmetastatic Renal Cell Cancer with Tumor Thrombus. J Urol 2017;198:810-6. [Crossref] [PubMed]
- Ciancio G, Livingstone AS, Soloway M. Surgical Management of Renal Cell Carcinoma with Tumor Thrombus in the Renal and Inferior Vena Cava: The University of Miami Experience in Using Liver Transplantation Techniques. Eur Urol 2007;51:988-94. [Crossref] [PubMed]
- Glazer AA, Novick AC. Long-term followup after surgical treatment for renal cell carcinoma extending into the right atrium. J Urol 1996;155:448-50. [Crossref] [PubMed]
- Granberg CF, Boorjian SA, Schaff HV, et al. Surgical management, complications, and outcome of radical nephrectomy with inferior vena cava tumor thrombectomy facilitated by vascular bypass. Urology 2008;72:148-52. [Crossref] [PubMed]
- Libertino JA, Zinman L, Watkins E. Long-term Results of Resection of Renal Cell Cancer with Extension into Inferior Vena Cava. J Urol 1987;137:21-4. [Crossref] [PubMed]
- Blute ML, Boorjian SA, Leibovich BC, et al. Results of inferior vena caval interruption by greenfield filter, ligation or resection during radical nephrectomy and tumor thrombectomy. J Urol 2007;178:440-5; discussion 444. [Crossref] [PubMed]
- Hyams ES, Pierorazio PM, Shah A, et al. Graft reconstruction of inferior vena cava for renal cell carcinoma stage pT3b or greater. Urology 2011;78:838-43. [Crossref] [PubMed]
- Abaza R. Initial series of robotic radical nephrectomy with vena caval tumor thrombectomy. Eur Urol 2011;59:652-6. [Crossref] [PubMed]
- Chopra S, Simone G, Metcalfe C, et al. Robot-assisted Level II–III Inferior Vena Cava Tumor Thrombectomy: Step-by-Step Technique and 1-Year Outcomes. Eur Urol 2017;72:267-74. [Crossref] [PubMed]
- Wang B, Huang Q, Liu K, et al. Robot-assisted Level III-IV Inferior Vena Cava Thrombectomy: Initial Series with Step-by-step Procedures and 1-yr Outcomes. Eur Urol 2020;78:77-86. [Crossref] [PubMed]
- Kaag MG, Toyen C, Russo P, et al. Radical nephrectomy with vena caval thrombectomy: a contemporary experience. BJU Int 2011;107:1386-93. [Crossref] [PubMed]
- Campbell SC, Lane, Brian R., and Pierorazio, Phillip M. Malignant Renal Tumors. In: Partin AW, Peters, Craig A., Kavoussi, Louis R., Dmochowski, Roger R., and Wein, Alan J., editor. Campbell Walsh Wein Urology. 12th ed.: Elsevier; 2019. p. 2133-84.
- Blute ML, Inman, Bryant. Vena Caval Thrombectomy. In: Smith J, Smith, Joseph, Howards, Stuart, and Preminger, Glenn editor. Hinman's Atlas of Urologic Surgery. 3rd ed.: Elsevier; 2012. p. 1025-37.
- Fonouni H, Mehrabi A, Soleimani M, et al. The need for venovenous bypass in liver transplantation. HPB (Oxford) 2008;10:196-203. [Crossref] [PubMed]
- Ciancio G, Shirodkar SP, Soloway MS, et al. Renal carcinoma with supradiaphragmatic tumor thrombus: avoiding sternotomy and cardiopulmonary bypass. Ann Thorac Surg 2010;89:505-10. [Crossref] [PubMed]
- Sarkar M, Prabhu V. Basics of cardiopulmonary bypass. Indian J Anaesth 2017;61:760-7. [Crossref] [PubMed]
- Nguyen HG, Tilki D, Dall'Era MA, et al. Cardiopulmonary Bypass has No Significant Impact on Survival in Patients Undergoing Nephrectomy and Level III-IV Inferior Vena Cava Thrombectomy: Multi-Institutional Analysis. J Urol 2015;194:304-8. [Crossref] [PubMed]
Cite this article as: Antoine SG, Pfeifer Z, Carroll AM, Reece TB, Lloyd GL. Management of locally advanced renal cell carcinoma. AME Med J 2021;6:5.

