
Narrative review of the prognostic significance of immune cells in the tumor microenvironment of stage I lung cancer
Introduction
Our discussion of the immune microenvironment (IME) in early lung cancer will include the importance of immune cells in the tumor IME. Not only is the presence of certain immune cells relevant, their exact location within the tumor microenvironment also appears to be significant: i.e., within the tumor itself, or in the surrounding stroma, as are the types of infiltrating immune cells [including lymphocytes—B-cells, T-cells (CD4+, CD8+, regulatory T-cells/T-reg), macrophages and neutrophils], the density of immune cells, and the proportion of pro-tumor and anti-tumor immune cells (1). This article will elaborate on each of these aspects of tumor infiltrating immune cells. We present the following article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-20-118/rc).
Methods
We performed a PubMed search using the following phrases “lung cancer”, “immune prognostic factors”, “stage I”, which provided 88 search results. We further limited the publication dates to the last 10 years: 2009-01-01–2019-12-31, which filtered the search results down to 74 (Figure 1). We further excluded articles whose primary focus was specifically stated to be advanced stage lung cancer, articles we were unable to get full access to, and articles that were not relevant to the topic at hand, e.g., articles focused on treatment, genetic analysis, non-lung cancer, etc. More relevant articles were found within the reference lists of the previously mentioned relevant articles. In the current review, we will focus on immune cells in the tumor microenvironment. In total we reviewed 54 articles, to obtain a robust summary of the current available knowledge on the IME associated with stage I and early-stage lung cancer.
Discussion
Tumor-infiltrating lymphocytes (TILs), non-specified
Most simple analysis of TILs is an assessment on routine hematoxylin and eosin stains (H&E). Such approach has demonstrated the prognostic value of TILs. An example is a study of 219 patients who underwent lobectomy for stage I non-small cell lung cancer (NSCLC). Tissue samples were stained by routine H&E stain and analyzed by blinded pathologists. The results showed that a moderate-to-severe lymphocyte infiltration of large (≥5 cm) stage I tumors (n=39) was associated with a lower disease-recurrence and a higher 5-year disease-free survival (DFS). Specifically, patients with no-to-low TILs had a 35.9% 5-year DFS, compared with 75.6% in patients with moderate-to-high TILs. Similarly, disease recurrence was noted as 60% and 21% in both groups of patients, respectively (2). Another study analyzed 783 stage I–III NSCLC samples by H&E and showed a significant increase in overall survival (OS) and DFS in patients whose tumors had intense lymphocytic infiltration. The 5-year OS in tumors with intense tumor lymphocyte infiltration and those with non-intense tumor lymphocyte infiltration was 59% and 40%, respectively (P=0.001) while 5-year DFS was 54% and 35% in both groups, respectively (P=0.001). These results were confirmed by similar observation in the validation data set (n=763). This association, although also present in resected adenocarcinoma (ADC) and squamous cell carcinoma (SCC), was greater in non-ADC and non-SCC histological subtypes which included large-cell, adenosquamous, sarcomatoid, basaloid, and unclassifiable NSCLC, in which there was an 88% decrease in the risk of death in patients with intense lymphocytic infiltration, compared with a 38% decrease in the risk of death in the SCC plus ADC groups combined (3). In stage IA NSCLC, similar trends have been shown by Horne et al., in which TILs were shown to infer a significant beneficial association on 5-year DFS in their study population, as well as in a subset group without angiolymphatic invasion. Five-year DFS was 87% in the TIL-positive (TIL+) group, compared with 73% in the TIL-negative (TIL−) group (P=0.011). Furthermore, while there was no statistical difference in locoregional recurrences between the TIL+ and TIL− groups, systemic recurrence was more common in the TIL− group (16.4%) than in the TIL+ group (6.4%; P=0.0295). In the subset of patients without angiolymphatic invasion, 5-year DFS was higher in the TIL+ group (93%), than in the TIL− group (79%); P=0.036 (4). Similar results were reported by Ruffini et al. in their immunohistochemistry (IHC) study of 1290 resected NSCLC tissues. In this study, a high TIL infiltration correlated with increased cumulative survival in stage I SCC patients (P=0.03), but not in stage I ADC patients (P=0.63), or in the general study population not stratified by histological subtype (5). In summary, TILs have been shown to be associated with prolonged OS and reduced disease recurrence. These studies (Table 1) demonstrate the prognostic value of immune response gained from simple H&E; however, they did not investigate specific subtypes of immune cells.
Table 1
| Author | Stages | n | Conclusion |
|---|---|---|---|
| Kilic et al. (2) | I | 219 | High infiltration is associated with: |
| Lower disease recurrence | |||
| Longer 5-year DFS | |||
| No-to-low TILs in large (>5 cm) stage I tumors was associated with a 35.9% 5-year DFS, and a 60% recurrence, compared with moderate-to-high TILs patients, in whom 5-year DFS was 75.6%, and recurrence rate was 21% | |||
| Brambilla et al. (3) | I–III | 783 | High infiltration is associated with: |
| Longer OS | |||
| Longer DFS | |||
| 5-year OS for intense lymphocyte-infiltrated tumors was 59%, compared with 40% in low-infiltrated tumors (P=0.001) | |||
| 5-year DFS in high-infiltration tumors was 54%, compared with 35% in the low-infiltration group (P=0.001) | |||
| Horne et al. (4) | IA | 273 | High infiltration is associated with: |
| Increased rate of 5-year DFS | |||
| Decreased systemic recurrence | |||
| 5-year DFS was 87% in the TIL+ patients, and 73% in the TIL- patients; P=0.011 | |||
| Systemic recurrence rate was 6.4% in the TIL+ group, and 16.4% in the TIL- group; P=0.0295 | |||
| Ruffini et al. (5) | I–IIIA | 1,290 | Increased TIL prevalence trended with increased survival in SCC (P=0.03), but not in other histological subtypes |
DFS, disease-free survival; TILs; tumor-infiltrating lymphocytes; OS, overall survival; SCC, squamous cell carcinoma.
CD8+ T-cells
CD8+ T-cells are referred to as the effector arm of the immune system as they are involved in the cell-mediated recognition and destruction of abnormal cells, including infected and cancer cells, by recognizing non-self-antigens expressed on major histocompatibility complex class I (MHC I) molecules on aforementioned abnormal cells (6). CD8+ TILs have been shown to infer a good prognosis in lung ADC. Laboratory experiments on murine and human cells have shown that T-cells can kill tumor cells (7). Schalper et al. found in their study of stage I–IV NSCLC in 2 independent groups using H&E and multiplex immunofluorescent staining that high CD8+ T-cell infiltration in the tumor microenvironment was independently associated with improved survival (Group 1: P=0.03, 95% CI: 0.280–0.968, HR: 0.533. Group 2: P=0.004, 95% CI: 0.385–0.842, HR: 0.576) (8). Another study on ADC showed that a high CD8+ T-cell infiltration as assessed by IHC was associated with a significantly improved 5-year OS; this survival advantage was even more pronounced when a high CD8+ infiltration was associated with a concurrently high mature dendritic cell infiltration (5-year OS: 45.4% with low CD8+ infiltration, 62.7% with high CD8+ infiltration, P=0.002; 64.4% with high CD8+ and high mature dendritic cells, P=0.00008) (9). These findings are supported by other studies, including that by Alifano et al., in which a high intratumoral CD8+ T-cell and mature dendritic cell infiltration, assessed by IHC, were associated with a significant improvement in 5-year OS, including in stage I–II lung cancers. Higher CD8+ infiltration was associated with a 63.3% 5-year OS, compared with 35.7% in low-infiltration tumors (P=0.0028); similarly, high mature dendritic cell infiltration was associated with a 5-year OS of 59.2%, compared with 40.2% in low infiltration tumors (P=0.0015) (10). Improved DFS and OS were demonstrated by Teng et al. in their study, also using IHC on stage I NSCLC tissue samples. DFS showed a positive correlation with increased CD8+ infiltration in both univariate (P=0.002, 95% CI: 0.217–0.714, HR: 0.393) and multivariate analyses (P=0.034, 95% CI: 0.053–0.892. HR: 0.218). Similar findings were shown for OS in univariate analysis (P=0.044, 95% CI: 0.259–0.982, HR: 0.505) although multivariate analysis only showed a trend between high CD8+ TILs and improved OS (P=0.070, 95% CI: 0.276–1.052, HR: 0.539) (11). Usó et al. assessed the presence of immune cells in 122 stage I–IIIA NSCLC tissue samples, using both IHC and gene expression by means of quantitative polymerase chain reaction (PCR). Both IHC and gene expression analyses showed that a high tumor CD8+ infiltration in NSCLC was associated with improved DFS and OS. Univariate analysis of IHC-derived results showed that a high CD8+ infiltration was associated with a median DFS 56.8 months, compared with a median DFS of 23 months in the low-infiltration group (P=0.026). Similarly, improved OS was shown in univariate analysis of IHC data, with high and low CD8+ infiltration being associated with a median OS of 73.9 months and 40.4 months, respectively (P=0.021). Univariate analysis of gene expression data also showed a similar positive correlation between high tumoral CD8+ infiltration and DFS (median DFS for high vs. low tumoral infiltration: 81.2 vs. 19.4 months; P<0.001) and OS (median OS for high vs. low CD8+ infiltration: 81.2 vs. 37.2 months; P<0.001). Univariate analysis of gene expression data showed that a high stromal CD8+ infiltration was significantly associated with improved OS, (median OS survival for high vs. low stromal CD8+ infiltration respectively: 74.3 months, 46.4 months, P=0.032), but not DFS (29.1 vs. 25.6 months; P=0.237). Multivariate analyses showed that tumoral CD8+ infiltration was an independent prognostic factor for OS and DFS, using both gene expression and IHC data (Gene expression: OS, P<0.001, 95% CI: 0.064–0.447, HR: 0.169; DFS, P<0.001, 95% CI: 0.120–0.507, HR: 0.247. IHC data analysis: OS, P=0.018, 95% CI: 0.175–0.850, HR: 0.386; DFS, P=0.004, 95% CI: 0.137–0.680, HR: 0.305) (12). Although several studies have shown CD8+ infiltration to be associated with a better prognosis, there are some studies with contradicting results. Mori et al. found in their IHC study of 128 stage I to IIIB NSCLC samples that tumoral CD8+ infiltration was not associated with survival, but that greater CD8+ infiltration was more associated with undifferentiated tumors than with well- or moderately-differentiated tumors (P<0.05) (13). Similar findings were also seen in a study by Ilie et al., in which IHC staining was performed on 632 stage I–III NSCLC tissue samples. Progressively higher CD8+ infiltration was seen as tumors became less differentiated. High infiltration in grade 1 to 4 tumors was seen in 43%, 52%, 54% and 65% of patients, respectively (14). These results are also supported by the study by Wakabayashi et al., in which CD8+ T-cells assessed by IHC in the tumor nests of 178 NSCLC tissues samples were found to be negative prognosticators for survival. CD8+ cells were more frequently found in poorly and undifferentiated tumor nests than in well-differentiated tumor nests (P<0.01); 5-year OS was 47% in high CD8+ infiltrated tumor nests, compared with 60% in low CD8+ infiltrated nests (P=0.03). When the data was stratified by histological subtype, the survival disadvantage associated with high CD8+ tumoral infiltration was maintained in ADC (P=0.02), but not in SCC (15). These prognostic findings were not seen for CD8+ cells in the tumor stroma. Increased CD8+ infiltration has been shown to be negatively correlated with decreased expression of tumor microenvironment programmed death ligand-1 (PD-L1), a transmembrane protein found on the surface of various cells of the immune system, including B- and T- lymphocytes, Natural Killer (NK) cells, activated monocytes, macrophages and dendritic cells, whose functions include regulating the immune response by effects that include promoting regulatory T-cell (T-reg) activity (16,17). Rashed et al. in their IHC analysis of various cell molecules in NSCLC, showed that tumors with low levels of CD8+ TILs were associated with greater PD-L1 positivity (75%), compared with high CD8+ TILs tumors, which expressed much lower PD-L1 positivity in the microenvironment (17.9%; P=0.009) (18). These findings suggest that the lack of CD8+ infiltration in the IME of lung cancer may be associated with decreased anti-tumoral response by the immune system. In summary, there appears to be a general notion that CD8+ T-cell infiltration is associated with improved prognosis. Variances between non-corroborating studies may be attributed to a number of reasons, including varying sample sizes, different IHC markers and techniques, lack of standardized cut-offs to define high-vs-low infiltration, and lack of studies investigating the functional status of these cells (Table 2).
Table 2
| Author | Stages | n | Conclusion |
|---|---|---|---|
| Teng et al. (11) | I | 126 | High CD8+ infiltration is associated with: improved DFS in univariate and multivariate analyses, and improved OS in univariate but not multivariate analysis |
| DFS in: | |||
| Univariate analysis (P=0.002, 95% CI: 0.217–0.714, HR: 0.393); 5-year DFS was ~40% in low-infiltration, vs. ~70% in high infiltration | |||
| Multivariate analyses (P=0.034, 95% CI: 0.053–0.892, HR: 0.218) | |||
| OS in: | |||
| Univariate analysis (P=0.044, 95% CI: 0.259–0.982, HR: 0.505); 5-year OS was ~60% in low-infiltration, vs. ~80% in high-infiltration | |||
| Multivariate analysis (P=0.070, 95% CI: 0.276–1.052, HR: 0.539) | |||
| Rashed et al. (18) | III–IV | 50 | High CD8+ infiltration is associated with lower expression of PD-L1 on TCs |
| 75% of low CD8+-infiltrated tumors expressed PD-L1, compared with 17.9% of high CD8+-infiltrated tumors; P=0.009 | |||
| Schalper et al. (8) | I–IV | 552 | Increased CD8+ infiltration is associated with improved survival in 2 independent groups; P=0.004, P=0.002 |
| Group 1: ~40% 5-year survival probability in low-infiltration, vs. ~60% in high-infiltration | |||
| Group 2: ~20% 5-year survival probability in low-infiltration, vs. ~50% in high-infiltration | |||
| Mansuet-Lupo et al. (9) | I–IV | 316 | High CD8+ infiltration is associated with an increased 5-year OS. 5-year OS for: |
| Low CD8+ infiltration: 45.4% | |||
| High CD8+ infiltration: 62.7%; P=0.002 | |||
| Alifano et al. (10) | I–IV | 303 | High CD8+ infiltration is associated with an increased 5-year OS than low CD8+ infiltration. 5-year OS for: |
| Low CD8+ infiltration: 35.7% | |||
| High CD8+ infiltration: 63.3%; P=0.0028 | |||
| Usó et al. (12) | I–IIIA | 122 | Increased CD8+ tumor infiltration is associated with: improved DFS and improved OS |
| Median DFS of 23 months in low-infiltration, vs. 56.8 months in high-infiltration; P=0.026 | |||
| Median OS of 40.4 months for low-infiltration, vs. 73.9 months in high-infiltration; P=0.021 | |||
| Increased CD8+ stromal infiltration is associated with: improved OS | |||
| Median OS of 46.4 months in low-infiltration, vs. 74.3 months in high-infiltration; P=0.032 | |||
| CD8+ tumoral infiltration is an independent prognostic factor for OS and DFS | |||
| Mori et al. (13) | I–IIIB | 128 | High CD8+ infiltration is more prevalent in undifferentiated/poorly differentiated tumors than in well- and moderately differentiated tumors (P=0.05) |
| Ilie et al. (14) | I–III | 632 | High CD8+ infiltration is more prevalent in poorly differentiated, than in well- and moderately differentiated tumors |
| Global chi-square test P=0.04 | |||
| CD8+ infiltration was much greater in grade 2-4 tumors than in grade 1 tumors (P=0.009) | |||
| Wakabayashi et al. (15) |
I–III | 178 | High CD8+ tumoral infiltration is associated with: |
| Poorly differentiated than well-differentiated tumors (P<0.01) | |||
| Decreased 5-year OS (5-year OS for high vs. low infiltration were 47% and 60%, respectively; P=0.03) | |||
| Mansuet-Lupo et al. (9) | I–IV | 316 | High infiltration by both CD8+ T-cells and mature dendritic cells is associated with improved survival |
| 5-year OS was: | |||
| 64.4% with high CD8+ and mature dendritic cell infiltration | |||
| 60.8% with high CD8+ and low mature dendritic cell, or low CD8+ and high mature dendritic cell | |||
| 36.6% with low CD8+ and low mature dendritic cell infiltration | |||
| P=0.00008 |
PD-L1, program death ligand 1; TC, tumor cells; DFS, disease-free survival; OS, overall survival; CI, confidence interval; HR, hazard ratio.
CD4+ T-cells
CD4+ T-cells have been described to assume one of two opposing functions, whereby one extreme is composed of CD4+ T-cells responsible for promoting CD8+ effector response, while the other end of the spectrum is composed of CD4+ cells that inhibit CD8+ cell response, and essentially play a regulatory role (i.e., regulatory T-cells) (19). Regulatory T-cells (T-regs) are a subset of CD4+ T-cells with high expression of CD25 and CD127 (19). It follows that the earlier mentioned CD4+ subset may likely play an important anti-tumor role, and the latter may be involved in tumorigenesis and progression (5). T-regs also contain the nuclear transcription factor, Foxp3, which is also used as a marker of this subset of lymphocytes known to suppress the host immune response (19). Mony et al. in their analysis of microarray data from the Gene Expression Omnibus (GEO), showed that the increased presence of non-regulatory CD4+ T-cells was associated with a higher non-recurrence (P=0.0001) in stage IA/IB ADC (20). Corroborating results were shown by Wakabayashi et al., in which the 5-year OS was higher in tumors with higher CD4+ infiltration in the cancer stroma (64%), compared with tumors with lower CD4+ stromal infiltration (43% P=0.04) (15). Usó et al. had concurring results but in the tumor nest, in which tumoral CD4+ T-cells were associated with better prognosis, and specifically, a longer OS and DFS in stage I–IIIA NSCLC (CD4+ high vs. low tumoral infiltration: median OS: 81.2 vs. 42.9 months, P=0.018; median DFS: 37.8 vs. 23 months, P=0.042) (12).
Regulatory T-cells
T-regs have been shown to be involved in suppressing the immune response (21,22); this effect is a favorable one in terms of preventing the development of autoimmune disorders, but may become adverse in other situations, as appears to be the case in lung cancer. In the study by Usó et al., the ratio of Foxp3 a marker of T-regs, to the total CD4+ T-cell was assessed; when combined as a ratio, a high stromal Foxp3-to-tumour-CD4+ expression inferred a significant reduction in OS (46.6 months) and DFS (19.4 months), compared with a low-stromal Foxp3-to-tumour-CD4+ ratio (OS: 81.2 months, P=0.012; DFS: 37.8 months, P=0.013). These results indicate that increasing the regulatory subset of CD4+ T-cells possibly shifts the immune dynamic towards tumor growth and progression (12). This disadvantageous effect of T-regs in the cancer IME has been shown in further studies. Mony et al. analyzed the patterns in immune composition associated with recurrence in NSCLC (specifically ADC), using microarray data derived from the GEO, and showed that a high presence of T-regs was associated with increased recurrence (P=0.0048); a low proportion of macrophages/monocytes was associated with increased non-recurrence (P=0.0047), and a greater ratio of T-reg-to-total-T-cell increased the probability of recurrence (P=0.0032) (20). These results are in harmony with the current knowledge of the functions of T-regs in diminishing the responses of effector T-cells, including their antitumoral activities (21,22). Studies have shown that a low stromal Foxp3 confers a prognostic advantage over a high stromal Foxp3, which is associated with a shorter DFS. Specifically, Suzuki et al. showed in their IHC study of 956 stage I lung ADC tissue samples that a higher 5-year DFS was associated with a low stromal Foxp3 presence (85%), compared with the group of patients with a high stromal Foxp3, which was associated with a 5-year DFS of 80% (P=0.043) (23,24). Furthermore, a high stromal CD3 (i.e., overall T-cell) infiltration became protective against a high stromal Foxp3, leading to a higher DFS. Five-year DFS in patients with a high stromal Foxp3-to-stromal CD3+ ratio was 77%, compared with patients with a low stromal Foxp3-to-stromal CD3+ ratio, who had a 5-year DFS of 85%; P=0.004 (23). The notion that increased T-regs in the tumor microenvironment is associated with tumorigenesis is further supported by another study that revealed that a high stromal Foxp3 infiltration and a high Foxp3/CD3+ ratio, as assessed by IHC analysis, were associated with increased tumor budding, as assessed by H&E staining. Tumor budding refers to the presence of lone tumor cells or small collections of less than 5 tumor cells, at the outer margin of the tumor, in the tumor stroma, indicative of increased tumor invasiveness and negative prognosis. This study showed that high-grade tumor budding correlated with increased stromal Foxp3 (P<0.001) and was associated with an increased cumulative recurrence incidence (CRI). High-grade tumor budding was seen in 41% of tumors with high stromal Foxp3 infiltration, compared with 26% of tumors with low stromal Foxp3 infiltration (P≤0.001). There were similar trends with tumoral Foxp3+ infiltration, but the findings were not significant (P=0.37). Tumors with high-grade budding were associated with a higher 5-year CRI of 32%, compared with low-grade budding (12%, P<0.001). This finding was reproduced in the validation dataset, in which 5-year CRI was 20% in the high-grade budding tumors, compared with 12% in the low-grade budding tumors; P=0.005 (25). Another study performed by IHC on resected stage I–III NSCLC tissues, showed that increased Foxp3+ tumor infiltration (>3 cells) was associated with worse DFS, compared with patients with low tumor Foxp3+ (<3 cells) infiltration (P=0.004). Furthermore, Foxp3 expression was an independent predictor of DFS following multivariate analysis, in which higher levels of Foxp3 T-cells were associated with shorter DFS (P<0.016, 95% CI: 1.38–21.07, HR: 5.38) (26). Foxp3 infiltration has also been shown to correlate with increased expression of nuclear estrogen-receptor-α in male lung ADC and subsequently increased risk of recurrence (27). A positive correlation has also been shown between Foxp3 infiltration and intratumoral cyclooxygenase expression (26). This could be explained by another study that showed that tumoral COX-2 induces Foxp3 expression, leading to increased activity of T-regs in lung cancer (28). This may, at least in part, explain the poorer prognosis in terms of DFS seen in the study by Shimizu et al. (26), since COX-2 elaborates prostaglandin E2 (PGE2), which increases the presence of T-regs, which in turn suppress the immune response against tumors (Figure 2). In the study by Shimizu et al. (26), poorer prognosis was more greatly highlighted in patients without nodal metastasis. Specifically, the DFS in node-negative patients with high Foxp3 infiltration was significantly worse than in node-negative patients with low-to-no Foxp3 infiltration (P<0.001); there was no significant difference in DFS between high and low Foxp3 infiltration in the node-positive group (P=0.688), possibly hinting that Foxp3 cells may play a more important role in the early-stage tumors (26). The finding that tumor-infiltrating Foxp3 cells are associated with worse prognosis in terms of recurrence has been reproduced by other studies (11,29-31). Yan et al. showed that high expression of Foxp3 was associated with shorter DFS (HR =1.336, P=0.031), and OS (HR =1.566, P=0.002) in stage I-IV NSCLC (31). Teng et al. in their study of stage I NSCLC revealed that Foxp3 T-regs were not independent prognosticators of survival, but rather low levels in combination with high CD8+ TILs, were associated with a longer DFS. Low-Foxp3/high-CD8+ TILs was associated with a better DFS than low-Foxp3/low-CD8+ (P=0.048) and high-Foxp3/low-CD8+ (P=0.017). However, this prognostic advantage of low-Foxp3 was attributed to the associated high levels of CD8+ TILs since there was no significant difference between patients with low-Foxp3/low-CD8+ and high-Foxp3/low-CD8+ (P=0.555), and also no difference between patients with high-Foxp3/high-CD8+ and low-Foxp3/high-CD8+ (P=0.501) (11). One mechanism by which the immunosuppressive effect of T-regs is maintained in lung cancer has been suggested to be via the transmembrane glycoprotein, glycoprotein A repetitions predominant (GARP), which maintains the regulatory functions of T-regs, and has been shown to be associated with advanced disease when expressed on Foxp3+ T-regs (32). In general, it appears that increased expression of T-regs in lung cancer is associated with worse prognosis, including shorter OS and DFS, and that ratios of these T-regs to other cells (CD3, CD4, and CD8) have prognostic value. On the other hand, increased non-T-reg CD4+ T-cell infiltration may be associated with improved OS and DFS (Table 3).
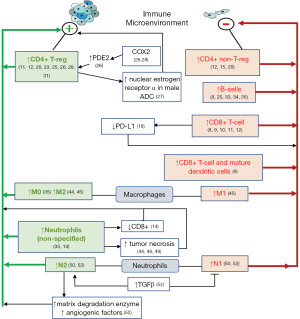
Table 3
| Immune cell | Author | Stages | n | Conclusion |
|---|---|---|---|---|
| CD4+ non-regulatory | Mony et al. (20) | IA/IB | 280 | High infiltration is associated with longer DFS (P=0.0001) |
| Wakabayashi et al. (15) | I–III | 178 | Higher stromal infiltration is associated with increased 5-year OS (64%), than lower stromal infiltration (5-year OS =43%; P=0.04) | |
| Usó et al. (12) | I–IIIA | 122 | High CD4+ infiltration is associated with: | |
| Longer DFS | ||||
| Longer OS | ||||
| Tumors with high vs. low CD4+ infiltration respectively had: | ||||
| Median OS: 81.2 vs. 42.9 months, P=0.018 | ||||
| Median DFS: 37.8 vs. 23 months, P=0.042 | ||||
| T-regs/Foxp3 | Mony et al. (20) | I | 280 | High prevalence of T-regs is associated with increased recurrence (P=0.0048) |
| Increased T-reg-to-total-T-cell ratio is associated with increased probability of recurrence (P=0.0032) | ||||
| Usó et al. (12) | I–IIIA | 122 | High stromal Foxp3-to-tumour-CD4+ expression is associated with a reduced OS and DFS | |
| OS for high vs. low ratio: 46.6 vs. 81.2 months; P=0.012 | ||||
| DFS for high vs. low ratio: 19.4 vs. 37.8 months; P=0.013 | ||||
| Suzuki et al. (23) | I | 956 | High stromal Foxp3 is associated with an increased risk of recurrence | |
| Low stromal Foxp3 is associated with a higher 5-year RFP (85%), compared with high stromal Foxp3, which was associated a lower 5-year RFP (80%). P=0.043 | ||||
| Shimizu et al. (26) | I–III | 100 | High stromal Foxp3 tumor infiltration is associated with a shorter DFS (P=0.004), and shown to be an independent prognostic factor for DFS (P<0.016, 95% CI: 1.38–21.07, HR: 5.38) | |
| Peterson et al. (29) | I | 64 | A high Foxp3-to-CD3 ratio is associated with shorter survival and was shown to be an independent prognosticator for survival following multivariate analysis. P=0.007, 95% CI: 1.8–38.4, HR: 8.2 | |
| Median survival for low Foxp3-to-CD3 ratio tumors was >72 months, compared with 53 months for high Foxp3-to-CD3 ratio tumors (P=0.0148) | ||||
| Teng et al. (11) | I | 126 | Low Foxp3 infiltration in combination with high CD8+ infiltration is associated with longer DFS | |
| Low Foxp3/high-CD8+ TILs was associated with a better DFS than low-Foxp3/low-CD8+ (P=0.048) and high-Foxp3/low-CD8+ (P=0.017) | ||||
| Kadota et al. (25) | I | 1,038 | High stromal Foxp3+ infiltration correlates with high-grade tumor budding, which correlates with increased risk of recurrence | |
| High-grade tumor budding was seen in in 41% of tumors with high stromal Foxp3 infiltration, compared with 26% of tumors with low stromal Foxp3 infiltration; P<0.001 | ||||
| Tumors with high-grade budding were associated with a higher 5-year cumulative recurrence incidence (CRI) of 32%, compared with low-grade budding (12%, P<0.001) | ||||
| Yan et al. (31) | I–IV | 107 | Higher Foxp3 expression in TILs is associated with shorter DFS (HR: 1.336, P=0.031) and OS (HR: 1.566, P=0.002) |
DFS, disease-free survival; OS, overall survival; T-reg, regulatory T-cell; RFP, recurrence-free probability; CI, confidence interval; HR, hazard ratio; TIL, tumor-infiltrating lymphocyte.
B-lymphocytes
The presence of B-cells in the tumor IME can be recognized by their markers which include CD19, CD20 and CD79A, amongst others. B-lymphocytes have been shown to be increased in lung ADCs that show an increased expression of TTC21A, a gene whose upregulation has been shown to be associated with favorable prognosis by Wang et al. (33). This data analysis study using data derived from The Cancer Genome Atlas (TCGA) specifically showed that a high expression of TTC21A was associated with longer OS (P=0.002). Increased TTC21A expression was an independent prognosticator of improved survival (P=0.036, 95% CI: 0.623–0.984, HR:0.783). B-cells were more prevalent in tumors with high expression of TTC21A than in tumors with low expression (P=0.035) (33). One of the mechanisms by which tumor infiltrating B-cells are thought to contribute to the favorable prognosis of lung cancers is by secreting tumor-specific antibodies that boost T-cell response (33). The antitumoral effects of B-lymphocytes have also been reproduced by other studies, including that by Mony et al. (20). This study identified 2 subsets of patients with the highest risk of recurrence. One of these subsets included those patients with a high T-reg and macrophage/monocyte presence, and the second subset of patients with highest recurrence were those whose tumors had a low plasma cell (antibody-secreting B-cells) and non-T-reg CD4+ T-cell levels (P=0.0041). Plasma cells were further shown to be inversely correlated with recurrence (P=0.007) (20). Pelletier et al. in their prospective study of 113 stage I–IV NSCLC samples using histological stains (H&E, period acid Schiff, and mucicarmine), flow cytometry and IHC, found a lack of significant relationship between survival and the various prognostic markers they assessed, including T-lymphocyte infiltration. However, the study discovered a significant independent prognostic relationship between tumor-margin B-cells (stained using CD20 marker), and survival (P=0.04). Multivariate analysis continued to show that the presence of peritumoral B-cell infiltration was associated with improved survival (P=0.04, 95% CI: 0.06–0.42, HR: 0.16). The prognostic advantage associated with tumoral B-cell infiltration was specifically evident in non-SCC patients, who had better survival than patients with the same non-SCC histology without B-cell infiltration (P<0.001, 95% CI at 8 years: 0.04–0.40). The presence of B-cell infiltration in SCC was not found to be a significant prognostic factor (34). The results of these studies support the notion that humoral immunity plays an important antitumoral role in lung cancer. The positive prognostic relationship between tumor infiltration by B-cells in NSCLC and survival has also been portrayed by other studies (8,35). Schalper et al. performed a study on stage I-IV NSCLC, using 2 independent groups from 2 separate institutions. B-cells were stained for by immunofluorescent techniques, using their CD20 marker. This study showed that increased B-cell infiltration was associated with improved survival; this finding was significant in group 1 (P=0.004, 95% CI: 0.323–0.817, HR; 0.523), but not group 2 (P=0.293, 95% CI: 0.643–1.236, HR: 0.887). In group 1, 5-year survival probability was approximately 40% and 60% in the low- and high-infiltration groups respectively. Multivariate analysis continued to show B-cell infiltration to be an independent favorable prognostic factor in the earlier group (P=0.003, 95% CI: 0.222–0.754, HR: 0.418) (8). All of these findings suggest that humoral immunity plays an important antitumoral role in the IME of lung cancer (Table 4).
Table 4
| Author | Stages | n | Conclusion |
|---|---|---|---|
| Wang et al. (33) | I–IV | 535 | Higher B-cell infiltration was associated with higher TTC21A expression, which was shown to be an independent prognosticator of survival |
| B-cells were more prevalent in tumors with high expression of TTC21A than in tumors with low expression (P=0.035) | |||
| Low levels of TTC21A were associated with shorter survival on univariate analysis (P<0.046, 95% CI: 0.633–0.996, HR: 0.794) | |||
| Low levels of TTC21A were shown to be independently associated with shorter survival in multivariate analysis (P=0.036, 95% CI: 0.623–0.984, HR: 0.783) | |||
| Mony et al. (20) | I | 280 | Tumors with a low plasma cell and non-T-reg CD4+ infiltration combined, are associated with an increased recurrence (P=0.0041) |
| High plasma cell infiltration is associated with decreased recurrence (P=0.007) | |||
| Pelletier et al. (34) | I–IV | 113 | High B-cell infiltration was shown to be associated with improved survival in multivariate analysis. P=0.04, 95% CI: 0.06–0.42, HR: 0.16 |
| Schalper et al. (8) | I–IV | 552 | Increased B-cell infiltration is associated with improved survival (P=0.004) |
| Univariate analysis: P=0.004, 95% CI: 0.323–0.817, HR: 0.523 | |||
| 5-year survival probability was approximately 40% and 60% in the low- and high-infiltration groups respectively | |||
| Multivariate analysis: P=0.003, 95% CI: 0.222–0.754, HR: 0.418 | |||
| Liu et al. (35) | ‘Early clinical stage’ (not specified) | 980 | Increased memory B-cell infiltration (but not plasma cells: P=0.942) was associated with improved survival in lung ADC; P=0.047, 95% CI: 0.55–0.97, HR: 0.74 |
| This survival advantage trended in the SCC group, but was not significant. Plasma cell, P=0.099; memory B-cell, P=0.372 |
CI, confidence interval; HR, hazard ratio; non-T-reg, non-regulatory T-cell; ADC, adenocarcinoma; SCC, squamous cell carcinoma.
Macrophages
Macrophages play different roles in the immune system depending on the functional category assumed by the macrophages. Based on this premise, macrophages may be divided into 1 of 3 types: M0 macrophages (so called “resting macrophages”), M1 macrophages, also known as classically activated macrophages, which assume pro-inflammatory and anti-tumorigenic roles, and M2 macrophages, known as alternatively activated macrophages, with anti-inflammatory and pro-tumorigenic properties (36). Based on this understanding, it may be inferred that certain subtypes of macrophages may be associated with better and others poorer prognosis in lung cancer; however, various studies have produced differing results that do not necessarily align with the basic understanding of the different functions of macrophages. These variances between studies might be explained by the fact that macrophages do not tend to wholly assume an M1 or M2 phenotype in vivo, while losing the phenotype of the other subset, but rather adapt more characteristics that allow them to be classified as either M1 or M2, while still retaining some characteristics of the other subset, albeit more poorly expressed (37). Markers commonly used to identify macrophages include cell surface markers, cytokines and transcriptional factors, examples of which, regardless of subset, include but are not limited to: CD14, CD16, CD68, CD86 and HLA-II (37). M1 may be identified by: CD80, IL-1R, TLR2, TLR4, IL-12p70, and others. M2 macrophages may be identified by: CD163, CD200R, MMR/CD206, TLR1, TLR8, IL-1R II and others (37-40). Liu et al. in their data analysis-based study of ADC and SCC data derived from TCGA revealed that M0 macrophages were associated with decreased OS (P=0.049, 95% CI: 1.01–1.81, HR: 1.35) in early-stage lung ADC, while neither M1 nor M2 was associated with prognosis (35). A higher density of CD68 [a marker applied to all macrophages (41)] has also been found to be associated with poor prognosis in other human cancers (42,43). In lung cancer, Carus et al. discovered in their study of NSCLC (n=335) that metastasis was significantly higher in patients with increased tumor nest CD163+ macrophage and stromal CD163+ macrophage density (P<0.05), however, no survival associations were found (44). CD163+ is a marker of M2 macrophages (41). This finding supports the notion that M2 macrophages, and not M1 macrophages are protumor (45). It appears that increased tumoral infiltration by macrophages, regardless of their subset, may be associated with worse prognosis; however, further research into the functions of the different phenotypes of macrophages in lung cancer, may be warranted (Table 5).
Table 5
| Author | Stages | n | Conclusion |
|---|---|---|---|
| Liu et al. (35) | ‘Early clinical stage’ (not specified) | 980 | High infiltration of M0 macrophages were associated with poorer OS (P=0.049, 95% CI: 1.01–1.81, HR: 1.35) |
| Neither M1 nor M2 macrophages were found to be prognostic for survival (P=0.139 and 0.877, respectively) | |||
| Suzuki et al. (23) | I | 956 | Low density of macrophages (non-specified) was inversely related to IL-12R/β2 expression (P=0.006), a positive prognostic factor that is positively correlated with longer DFS (5-year DFS in tumors with low vs. high expression of IL-1212R/β2 was 80% and 90%, respective; P=0.026) |
| Carus et al. (44) | I–IIIA | 335 | Increased M2 macrophages in the tumor nest and stroma (denoted by the CD163 marker) was associated with metastasis (P<0.05), but showed no other prognostic associations |
OS, overall survival; CI, confidence interval; HR, hazard ratio; IL, interleukin; DFS, disease-free survival.
Neutrophils
Neutrophils are effector cells of the innate immune system, with important roles in acute and chronic inflammation, as well as adaptive immunity. They are identified by high expression of markers such as CD11, CD13, CD15, CD63, CD66, myeloperoxidase, and others (46,47). Liu et al. showed in their study using data from TCGA that increased neutrophilic infiltration in SCC was associated with decreased survival (P=0.009, 95% CI: 1.01–1.75, HR: 1.33); similar findings were not found in lung ADC (35). Carus et al. showed that intra-tumoral neutrophil (CD66+) density showed a positive correlation with T-stage (P=0.025), degree of necrosis (P<0.0001), and a greater association with SCC than ADC (P<0.05); however, no associations with OS or DFS were found (44). Although this study did not find any direct correlation between neutrophils and prognosis in terms of recurrence and survival, other studies have shown neutrophil-mediated tumor necrosis to be associated with poorer prognosis. Park et al. showed in their retrospective analysis of stage IA NSCLC, using H&E-stained tumor samples, that tumor necrosis was associated with a shorter 5-year OS and shorter 5-year DFS. In necrosis-negative samples, 5-year OS and DFS were 94.8% and 92.1% respectively, while OS and DFS findings for necrosis-positive patients were 86.2% (P=0.04) and 78.9% (P=0.016), respectively (48). Swinson et al. in their study on stage I–III NSCLC showed an increased hazard ratio (HR) when extensive tumor necrosis was present (HR: 1.76), compared with no-to-limited tumor necrosis (HR: 1, 95% CI: 1.24–2.50, P=0.0016) (49). This study also analyzed H&E-stained tumor samples. Samples were stratified according to the degree of necrosis, where samples with a score of 0 had no necrosis and more extensive necrosis was associated with higher scores. The hazard ratio (HR) for samples scored 0-2 was 1, while samples scoring 3–5 had a HR of 1.76 (95% CI: 1.24–2.50; P=0.0016) (49). The findings of these aforementioned studies suggest that neutrophilic infiltration may be associated with fast-growing tumors that outgrow their blood supply, with resulting tumor necrosis. Nevertheless, studies have shown that neutrophils are polarized into one of 2 phenotypes, similar to macrophages. Neutrophils may either assume the N1 or N2 phenotype, where the former has been shown to be involved in mediating antitumor response through mechanisms such as increasing intratumoral CD8+ T-cells, and the latter (N2) has been shown to be protumor and involved in depleting intratumoral CD8+ T-cells (50). TGF-β has been shown to be implicated in neutrophil polarization. This immunosuppressive cytokine is not only involved in suppressing CD8+ T-cell action (51), but also in steering the polarization of neutrophils towards the N2 phenotype, while blocking the elaboration of N1 neutrophils. The N2 neutrophilic phenotype has been suggested to mediate its protumor effects by causing the release of matrix-degrading enzymes and angiogenic factors, which will subsequently promote metastatic features and increased tumoral growth (52). Piccard et al. have provided a good summary of the mechanisms of the antitumor and protumor activities of the N1 and N2 neutrophilic subsets, respectively (53). This knowledge of the proposed phenotypes of neutrophils indicates that antitumoral response could be achieved by either stimulating the N1 phenotype or blocking the N2 phenotype. Fridlender et al. have suggested that treatment-naïve tumors are likely to be infiltrated by N2 neutrophils, meaning that depletion of these neutrophils will have an antitumor response, while tumors exposed to some form of immunologic therapy acquire N1-polarised neutrophils, indicating that neutrophil depletion in these tumors will likely lead to a protumor response (50). Ilie et al. found in their study of stage I-III NSCLC specimen using IHC, that a high intratumoral CD66b+ cells (i.e., neutrophils) correlated with low levels of CD8+ cells (P=0.016) (14). This study also revealed that high intratumoral infiltration by CD66+ cells was associated with increased rate of recurrence. Median recurrence rate for high CD66+ tumoral infiltration and low CD66+ infiltration was 36 and 51 months, respectively (P=0.002). Median OS for high vs. low CD66+ tumoral infiltration was 54 months and 57 months, respectively (P=0.088) (14). Furthermore, a high intratumoral neutrophil-to-lymphocyte ratio (NLR) was associated with decreased OS and increased rate of recurrence (median survival was 43 months for NLR <1, and 34 months for NLR >1; P<0.0001) (14). This may possibly indicate that a high tumoral neutrophil infiltration may blunt any potential CD8+ effector antitumoral effects. It has been suggested that tumors secrete chemotactic chemicals that increase the presence of neutrophils in tumors; these neutrophils appear to promote tumor growth rather than regression. This leaves the question as to whether tumors recruit N2 neutrophils, or non-N2 neutrophils are recruited and subsequently converted into neutrophils with protumoral N2 capabilities. Some studies have shown that tumor-associated neutrophils (TANs) in early lung cancer do not demonstrate suppression of effector T-cells, and were even shown to stimulate T-cells in vitro (54). This suggests that the second theory might be applicable in early-stage lung cancer. The knowledge of the functions of TANs in the IME provides possible therapeutic targets that require further research (Table 6).
Table 6
| Author | Stages | n | Conclusion |
|---|---|---|---|
| Liu et al. (35) | ‘Early clinical stage’ (not specified) | 980 | Increased neutrophilic infiltration in SCC is associated with decreased survival (P=0.009, 95% CI: 1.01–1.75, HR: 1.33); similar findings were not found in lung ADC (P=0.343) |
| Carus et al. (44) | I–IIIA | 335 | Increased tumoral, peritumoral and stromal neutrophilic infiltration are significantly associated with a higher degree of tumor necrosis (P<0.0001) |
| Park et al. (48) | IA | 201 | Tumor necrosis was associated with a shorter DFS in stage IA NSCLC patients |
| In necrosis-negative samples, 5-year OS and DFS was 94.8% and 92.1% respectively, while values for necrosis-positive patients were 86.2% (P=0.04) and 78.9% (P=0.016), respectively | |||
| Swinson et al. (49) | I–III | 178 | A greater degree of necrosis is associated with an increased HR |
| Tumors with a low degree of necrosis had a HR of 1, while tumors with more extensive necrosis had a HR of 1.76 (95% CI: 1.24–2.50; P=0.0016) | |||
| Ilie et al. (14) | I–III | 632 | High neutrophil (CD66+) infiltration, correlated with low CD8+ infiltration (P=0.016) |
| High intratumoral neutrophil (CD66+) infiltration was associated with shorter OS and increased recurrence rate | |||
| A high neutrophil-to-lymphocyte ratio was associated with worse survival | |||
| Median recurrence rate for high CD66+ infiltration and low CD66+ infiltration was 36 and 51 months, respectively; P=0.002 | |||
| Median OS for high vs. low CD66+ tumoral infiltration was 54 months and 57 months, respectively; P=0.088 | |||
| Median survival was 43 months for a neutrophil-to-lymphocyte ratio (NLR) <1, and 34 months for NLR >1; P<0.0001 |
SCC, squamous cell carcinoma; CI, confidence interval; HR, hazard ratio; ADC, adenocarcinoma; DFS, disease-free survival; OS, overall survival.
Conclusions
The general consensus from our review is that T-cell (non-specified) infiltration in NSCLC is associated with improved outcome and may play an important role in preventing distant metastasis. A high CD8+ T-cell infiltration of the tumor and its stroma appears to support survival and stave off disease recurrence. High infiltration by non-regulatory CD4+ T-cells appears to have similar antitumoral effects as CD8+ T-cells. Conversely, CD4+ T-regs seem to have an opposite effect, in that they have been shown to promote tumorigenesis and tumor progression. B-lymphocytes have been suggested to play an antitumoral role in lung cancer, possibly by elaborating antitumor antibodies that aid the immune system in tumor destruction. Macrophages appear to be associated with poor survival and increased recurrence; however, further studies may be required to further assess if the specific subsets of macrophages (M0, M1, and M2) have different prognostic effects in lung cancer. Neutrophils, similar to macrophages have been shown to be capable of assuming one of 2 polarized forms (N1, N2), with the N1 form being associated with an antitumor response, and the N2 form being associated with protumoral properties. Several studies have shown increased neutrophilic infiltration in the tumor IME to be associated with poorer prognosis, while another study showed that neutrophils in early lung cancer possess antitumoral properties, suggesting that polarization from antitumoral to protumoral neutrophils may occur with progression of lung cancer; more research may be warranted in this respect. The knowledge of the roles of immune cells in the tumor IME provides opportunities for potential therapeutic targets as well as clinical prognostication. Further research is still needed to clarify the roles of some cells and/or their subsets in the IME and their clinical usefulness as prognostic indicators (Figure 2, Table 7).
Table 7
| Effect in the IME | Cell | Author |
|---|---|---|
| Antitumor | CD8+ T-cell | Schalper et al. (8) Mansuet-Lupo et al. (9) Alifano et al. (10 Teng et al. (11) Usó et al. (12) Rashed et al. (18) |
| CD4+ non-T-regulatory T-cell | Usó et al. (12) Wakabayashi et al. (15) Mony et al. (20) |
|
| B-lymphocytes | Schalper et al. (8) Mony et al. (20) Pelletier et al. (34) Liu et al. (35) Wang et al. (33) |
|
| CD8+ T-cell | Mori et al. (13) Ilie et al. (14) Wakabayashi et al. (15) |
|
| Protumor | T-regulatory/Foxp3 T-cells | Teng et al. (11) Usó et al. (12) Mony et al. (20) Suzuki et al. (23) Kadota et al. (25) Shimizu et al. (26) Petersen et al. (29) Yan et al. (31) |
| Macrophages | Suzuki et al. (23) Liu et al. (35) Carus et al. (44) |
|
| Neutrophil | Ilie et al. (14) Liu et al. (35) Carus et al. (44) Park et al. (48) Swinson et al. (49) |
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Immune Response in Lung Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-20-118/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-118/coif). The series “Immune Response in Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. KS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of AME Medical Journal. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Kilic A, Landreneau RJ, Luketich JD, et al. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res 2011;167:207-10. [Crossref] [PubMed]
- Brambilla E, Le Teuff G, Marguet S, et al. Prognostic effect of tumor lymphocytic infiltration in resectable non-small-cell lung cancer. J Clin Oncol 2016;34:1223-30. [Crossref] [PubMed]
- Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res 2011;171:1-5. [Crossref] [PubMed]
- Ruffini E, Asioli S, Filosso PL, et al. Clinical Significance of Tumor-Infiltrating Lymphocytes in Lung Neoplasms. Ann Thorac Surg 2009;87:365-71. [Crossref] [PubMed]
- Flaherty D. Immunology for Pharmacy. Immunology for Pharmacy. Mosby, 2012:162-8.
- Crowther MD, Dolton G, Legut M, et al. Genome-wide CRISPR-Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat Immunol 2020;21:178-85. [Crossref] [PubMed]
- Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst 2015;107:dju435. [Crossref] [PubMed]
- Mansuet-Lupo A, Alifano M, Cuchet NP, et al. Intratumoral immune cell densities are associated with lung adenocarcinoma gene alterations. Am J Respir Crit Care Med 2016;194:1403-12. [Crossref] [PubMed]
- Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS one 2014;9:e106914. [Crossref] [PubMed]
- Teng F, Meng X, Wang X, et al. Expressions of CD8+TILs, PD-L1 and Foxp3+TILs in stage I NSCLC guiding adjuvant chemotherapy decisions. Oncotarget 2016;7:64318-29. [Crossref] [PubMed]
- Usó M, Jantus-Lewintre E, Bremnes RM, et al. Analysis of the immune microenvironment in resected non-small cell lung cancer: The prognostic value of different T lymphocyte markers. Oncotarget 2016;7:52849-61. [Crossref] [PubMed]
- Mori M, Ohtani H, Naito Y, et al. Infiltration of CD8+ T cells in non-small cell lung cancer is associated with dedifferentiation of cancer cells, but not with prognosis. Tohoku J Exp Med 2000;191:113-8. [Crossref] [PubMed]
- Ilie M, Hofman V, Ortholan C, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer 2012;118:1726-37. [Crossref] [PubMed]
- Wakabayashi O, Yamazaki K, Oizumi S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci 2003;94:1003-9. [Crossref] [PubMed]
- Jing W, Li M, Zhang Y, et al. PD-1/PD-l1 blockades in non-small-cell lung cancer therapy. Onco Targets Ther 2016;9:489-502. [Crossref] [PubMed]
- Shimanovsky A, Phuong L, Ryan J, et al. Immuno-oncology in lung cancer: A focus on anti-PD-1 and anti-CTLA-4 therapy in NSCLC. In: Dammacco F; Silvestris F. editors. Oncogenomics: From Basic Research to Precision Medicine. Academic Press, 2019:487-97.
- Rashed HE, Abdelrahman AE, Abdelgawad M, et al. Prognostic significance of programmed cell death ligand 1 (PD-L1), CD8+ tumor-infiltrating lymphocytes and p53 in non-small cell lung Cancer: An immunohistochemical study. Turk Patoloji Derg 2017;1:211-22. [Crossref] [PubMed]
- Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 2006;203:1693-700. [Crossref] [PubMed]
- Mony JT, Schuchert MJ. Prognostic implications of heterogeneity in intra-tumoral immune composition for recurrence in early stage lung cancer. Front Immunol 2018;9:2298. [Crossref] [PubMed]
- Woo EY, Yeh H, Chu CS, et al. Cutting Edge: Regulatory T Cells from Lung Cancer Patients Directly Inhibit Autologous T Cell Proliferation. J Immunol 2002;168:4272-6. [Crossref] [PubMed]
- Ganesan A-P, Johansson M, Ruffell B, et al. Tumor-Infiltrating Regulatory T Cells Inhibit Endogenous Cytotoxic T Cell Responses to Lung Adenocarcinoma. J Immunol 2013;191:2009-17. [Crossref] [PubMed]
- Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage i lung adenocarcinoma: Tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol 2013;31:490-8. [Crossref] [PubMed]
- Kadota K. Nitadori J ichi, Adusumilli PS. Prognostic value of the immune microenvironment in lung adenocarcinoma. Oncoimmunology 2013;2:e24036. [Crossref] [PubMed]
- Kadota K, Yeh YC, Villena-Vargas J, et al. Tumor Budding Correlates With the Protumor Immune Microenvironment and Is an Independent Prognostic Factor for Recurrence of Stage I Lung Adenocarcinoma. Chest 2015;148:711-21. [Crossref] [PubMed]
- Shimizu K, Nakata M, Hirami Y, et al. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol 2010;5:585-90. [Crossref] [PubMed]
- Kadota K, Eguchi T, Villena-Vargas J, et al. Nuclear estrogen receptor-a expression is an independent predictor of recurrence in male patients with pT1aN0 lung adenocarcinomas, and correlates with regulatory T-cell infiltration. Oncotarget 2015;6:27505-18. [Crossref] [PubMed]
- Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+CD25+ T regulatory cell activities in lung cancer. Cancer Res 2005;65:5211-20. [Crossref] [PubMed]
- Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 2006;107:2866-72. [Crossref] [PubMed]
- Hanagiri T, Fukumoto M, Koyanagi Y, et al. Regulatory T-Cells and micrometastasis in lymph nodes of stage I NSCLC. Anticancer Res 2014;34:7185-90. [PubMed]
- Yan X, Jiao SC, Zhang GQ, et al. Tumor-associated immune factors are associated with recurrence and metastasis in non-small cell lung cancer. Cancer Gene Ther 2017;24:57-63. [Crossref] [PubMed]
- Jin H, Sun L, Tang L, et al. Expression of GARP is increased in tumor-infiltrating regulatory T cells and is correlated to clinicopathology of lung cancer patients. Front Immunol 2017;8:138. [Crossref] [PubMed]
- Wang W, Ren S, Wang Z, et al. Increased expression of TTC21A in lung adenocarcinoma infers favorable prognosis and high immune infiltrating level. Int Immunopharmacol 2020;78:106077. [Crossref] [PubMed]
- Pelletier MP, Edwardes MDDB, Michel RP, et al. Prognostic markers in resectable non-small cell lung cancer: A multivariate analysis. Can J Surg 2001;44:180-8. [PubMed]
- Liu X, Wu S, Yang Y, et al. The prognostic landscape of tumor-infiltrating immune cell and immunomodulators in lung cancer. Biomed Pharmacother 2017;95:55-61. [Crossref] [PubMed]
- Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res 2016;39:1588-96. [Crossref] [PubMed]
- Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011;9:216. [Crossref] [PubMed]
- Chávez-Galán L, Olleros ML, Vesin D, et al. Much more than M1 and M2 macrophages, there are also CD169+ and TCR+ macrophages. Front Immunol 2015;6:263. [PubMed]
- Duluc D, Delneste Y, Tan F, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007;110:4319-30. [Crossref] [PubMed]
- Rőszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm 2015;2015:816460. [Crossref] [PubMed]
- Yu X, Guo C, Fisher PB, et al. Scavenger Receptors: Emerging Roles in Cancer Biology and Immunology. Adv Cancer Res 2015;128:309-64. [Crossref] [PubMed]
- Jézéquel P, Campion L, Spyratos F, et al. Validation of tumor-associated macrophage ferritin light chain as a prognostic biomarker in node-negative breast cancer tumors: A multicentric 2004 national PHRC study. Int J Cancer 2012;131:426-37. [Crossref] [PubMed]
- Mahmoud SMA, Lee AHS, Paish EC, et al. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol 2012;65:159-63. [Crossref] [PubMed]
- Carus A, Ladekarl M, Hager H, et al. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: No immediate impact on patient outcome. Lung Cancer 2013;81:130-7. [Crossref] [PubMed]
- Allavena P, Sica A, Garlanda C, et al. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 2008;222:155-61. [Crossref] [PubMed]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159-75. [Crossref] [PubMed]
- Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997;89:3503-21. [Crossref] [PubMed]
- Park SY, Lee HS, Jang HJ, et al. Tumor necrosis as a prognostic factor for stage IA non-small cell lung cancer. Ann Thorac Surg 2011;91:1668-73. [Crossref] [PubMed]
- Swinson DEB, Jones JL, Richardson D, et al. Tumour necrosis is an independent prognostic marker in non-small cell lung cancer: Correlation with biological variables. Lung Cancer 2002;37:235-40. [Crossref] [PubMed]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009;16:183-94. [Crossref] [PubMed]
- Suzuki E, Kim S, Cheung HK, et al. A novel small molecule inhibitor of TGFβ type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res 2007;67:2351-9. [Crossref] [PubMed]
- Shojaei F, Singh M, Thompson JD, et al. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A 2008;105:2640-5. [Crossref] [PubMed]
- Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 2012;82:296-309. [Crossref] [PubMed]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 2014;124:5466-80. [Crossref] [PubMed]
Cite this article as: Akpoviroro O, Suzuki K. Narrative review of the prognostic significance of immune cells in the tumor microenvironment of stage I lung cancer. AME Med J 2021;6:37.


