
Prognostic significance of peripheral blood immune response in early-stage non-small cell lung cancer: a narrative review
Introduction
The majority (80–85%) of patients with non-small cell lung cancer (NSCLC), the predominant form of lung cancer, present with locally advanced or metastatic disease at diagnosis (1,2). Even though earlier diagnosis with increased low-dose CT screening by the National Lung Screening Trial has lowered mortality rates by 20% (3), 5-year survival rates remain low for stage I at 77–92%, stage II at 53–60%, and stage III at 13–36% (4). Overall 5-year survival rate for NSCLC is <21% in the US (5).
Recurrence even after curative-intent surgery, the current standard of care, is at 24% in stage 1 NSCLC and 50% overall (2,4). Even with the advent of adjuvant chemotherapy and stereotactic radiotherapy, there remains a crucial need for a more robust understanding of the immune response in lung cancer in order to develop well-targeted immunotherapeutic strategies and improve patient outcomes. While the tumor-associated immune response plays an important role in tumor progression and aggressiveness (6), this response often extends beyond the tumor microenvironment into the peripheral blood (7) and is responsible for systemic symptoms common in cancer patients. Measuring immune markers in peripheral blood provides an opportunity to study the systemic inflammatory state in response to cancer as well as provide prognostic information without the need to access the tumor microenvironment.
Since lung cancer is a heterogeneous disease, there exists a possibility for a mosaic of cellular profiles to exist inside the primary tumor of a single patient. Further, metastases may be dominated by a distinct clonal subtype of the primary tumor; multiple organ systems have the potential to be affected by cells with distinct molecular characteristics. Therefore, there is an added utility in examining factors present in peripheral blood as they may accurately represent the molecular profile of the metastatic disease when compared to the cellular environment found from primary tumor sampling. As well, the biomarkers examined may be collected from routine blood draws, making them an attractive option due to their less invasive and inexpensive nature.
In this review, we have synthesized findings from published studies on (I) the involvement of peripheral blood in the complete immune response to NSCLC and (II) identified prognostic peripheral immune markers in the peripheral blood of early-stage NSCLC. We present the following article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-20-122/rc).
Methods
A literature search was performed using PubMed/Medline. Search criteria included human studies conducted between January 2000 to May 2020, and articles written in English. Initial search terms included peripheral blood biomarkers, prognostic, NSCLC. More targeted searches were conducted based on the results of our initial inquiry, including immune cell, lymphocyte response, cytokines, etc. Additional studies were identified based on reference lists of the studies examined.
Immune cells in peripheral blood
T lymphocytes
CD3+/CD4+ “helper” and CD8+ “cytotoxic” T cells play an important role in both identifying cancer cells and carrying out immune-mediated cell death. In the tumor microenvironment, it has been demonstrated that high CD3+ and CD8+ tumor infiltrating lymphocytes were associated with longer survival in NSCLC patients (8).
While patients could benefit from increased lymphocyte-mediated cell death in the tumor microenvironment, it is less certain whether peripheral lymphocyte activity plays a role in the early stages of cancer as part of the peripheral immune response. However, peripheral helper and cytotoxic T cells may augment the activity of the tumor microenvironment through cytokine signaling. Cytokines produced by T cells such as IL-1, IL-6, and TNF-alpha have been implicated as prognostic serum biomarkers for NSCLC (9).
Kobayashi and colleagues found that low peripheral lymphocyte count was an indicator of poor prognosis for NSCLC (10), most probably due to overexpression of Tregs in the peripheral blood. They examined 237 patients with node-negative NSCLC and found lymphopenia to be associated with poor prognosis (HR =3.84, 95% CI: 1.83–8.08, P<0.001) and recurrence of tumor (P=0.01). Higher levels of peripheral helper and cytotoxic T cells could minimize the effects of T cell exhaustion, where individual T cells undergo progressive dysfunction in the setting of a chronic inflammatory state caused by cancer. Reuben and colleagues demonstrate this by comparing T cell density in peripheral blood, tumor adjacent lung and tumor microenvironment in early-stage NSCLC patients (11). High T cell density in peripheral blood was associated with longer overall survival (OS) (HR =0.38–0.98, P=0.04), while high T cell density in tumor adjacent lung tissue was associated with shorter OS (HR =1.09–2.14, P=0.01) due to potential T cell exhaustion. This finding suggests that higher T cell density in peripheral blood could replace dysfunctional T cells near the tumor and ensure continuous immune response.
In contrast, high peripheral total lymphocyte count has also been found to be an independent predictor for increased recurrence. Sulibhavi and colleagues performed a retrospective review on 103 patients undergoing surgery for stage I NSCLC and found a 5-year recurrence-free survival (RFS) of 70% in patients with lymphocytosis vs. 96% in patients with low lymphocytes (P=0.003) (12). These contradictory findings can best be explained by the lack of differentiation between pro-tumor and anti-tumor subtypes of T cells when investigating this relationship. Peripheral T cell levels during tumor immune response may have a downstream effect on regulatory T cell overexpression as well as a direct contribution to immune activity in the tumor microenvironment.
T-regulatory cells
Regulatory T cells, known as Tregs, are a subset of T cells known for their role in immune surveillance and immunosuppression. They primarily act by downregulating induction and proliferation of CD4+ and CD8+ T cells via production of inhibitory cytokines such as TGF-β, IL-35, and IL-10. In addition, Treg activity correlates with cyclooxygenase-2 expression and increased tumor recurrence (13). Previous studies have found an association between increased CD4+CD25+ Tregs and immunosuppression as well as tumor progression in NSCLC, suggesting that Tregs may contribute to immune dysfunction in cancer patients (14). Increased numbers of CD4+CD25+FOXP3+ Tregs were found in the peripheral blood of stage I NSCLC patients (15,16). When co-cultured with activated CD4+ T cells, Treg proliferation and cytokine secretion were potently suppressed (15). As NSCLC progresses in stage, there is a significant increase in CD4+CD25+FOXP3+ Tregs (16). However, Meloni and colleagues found no significant increase in CD4+CD25+ Tregs in subsequent cancer stages after an initial increase from control to stage I (17). The initial increase in Tregs in NSCLC may have functional significance in immunosuppression since it resulted in decreased proliferation of CD4+ T cells compared to healthy controls (17). The association of increased peripheral blood CD4+CD25+FOXP3+ Tregs with advancing tumor stage and low CD4+ T cell count in stage I implies a significant role for Treg in the peripheral immune response of NSCLC.
Natural killer (NK) cells
NK cells are important players in the innate immune response by recognizing foreign or abnormal host cells lacking surface major histocompatibility complex class I (MHC-I). There are two major subtypes of NK cells: (I) CD56dim CD16+ NK cells are the most prevalent in blood and are known for their cytotoxicity and (II) CD56dim CD16– NK cells, on the other hand, regulate the immune response by secreting cytokines including IFN-gamma (18). NK cells have a fundamental role in tumor surveillance and in modulating the adaptive anti-tumor response by recognizing common ligands with the natural cytotoxicity-triggering receptors (NCRs). Picard and colleagues investigating the role of NK cells in NSCLC have indicated that CD56dim CD16+ NK cells are present at lower serum levels whereas CD56dim CD16– NK cells are at higher levels compared to healthy controls (18). Higher CD56dim CD16– NK cells correlated with significantly higher expression of two angiogenic markers, vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) (19). Moreover, a significant downregulation of the NKp46, a major NCR, was reported in NSCLC patients and in other cancers (18). These results are supported by an in vitro study that found a consistent decrease in NK cytotoxicity in NSCLC sera that was further exacerbated by chemotherapeutic treatment (20). NSCLC patients also exhibited higher serum levels of soluble human leukocyte antigen class I (sHLA-I) and G (sHLA-G), well-known immunosuppressors. Increased sHLA-I and sHLA-G levels were correlated with significantly poor prognosis, tumor progression and shorter survival (21).
Myeloid-derived suppressor cells (MDSCs)
MDSCs, commonly defined as granulocytic (CD14– CD11b+) or monocytic (Mo) (CD14+HLA-DR–/low) populations in humans, are a heterogeneous cell population known to have effects in both suppression of adaptive immunity and regulation of innate immunity via cytokine production of macrophages (22). In pathological stress such as cancer, the population of immature myeloid cells (IMCs) expands due to release from bone marrow prior to maturation and a partial block of the normal differentiation into mature myeloid cells (23). This upregulation of IMCs can lead to the increased expression of immune suppressive factors such as arginase 1, inducible nitric oxide synthase (NOS) as well as an increase in production of nitric oxide (NO) and reactive oxygen species (ROS).
Studies have demonstrated the increased presence of MDSCs in peripheral blood of patients with NSCLC (24), particularly those that express the following phenotypes: CD34+, CD33+, CD15–, and CD13+. Previous studies have shown that increased numbers of IMCs can play a role in suppressing antitumor responses as well as strongly inhibiting proliferation of CD4+ and CD8+ T cells (25), particularly in late-stage cancers (23,26). This relationship seems to hold true even in early-stage NSCLC as characterized by a 2012 peripheral blood monocyte (PBMC) gene expression study that found expression of 21 myeloid-specific genes to be negatively associated with survival (7).
Both MDSC subtypes, circulating CCR5+ Mo-MDSC (27) and total polymorphonuclear (PMN)-MDSCs (23,27), have been found to be significantly increased in resectable NSCLC compared to healthy controls, which correlated with reduced RFS. Of note, squamous cell carcinoma has been associated with higher levels of PMN-MDSCs than adenocarcinoma (27). With tumor progression and advancement of stage, the amount of circulating PMN-MDSCs is expected to increase.
In 2016, Pogoda and colleagues were the first to examine the distribution of Mo-MDSCs and monocytes/macrophages across various sampling locations of NSCLC patients (28). Mo-MDSCs were found to be of higher prevalence in the peripheral blood of NSCLC and concurrently found a higher prevalence of monocytes/macrophages in the lymph nodes and tumor tissues (27,28). Mo-MDSCs in the peripheral blood also expressed higher levels of immunosuppressive cytokines (TGF-β, IL-1β, and TNF) than those found in the tumor or nodal environment. These findings are consistent with the notion that Mo-MDSCs generally promote tumor growth through their immunosuppressive activity and that they may differentiate into monocytes/macrophages in the tumor microenvironment.
Cytokines
Numerous cytokines such as IL-4, IL-6, IL-10, IL-13 and TGF-β have been extensively studied for their immunosuppressive role in oncogenesis and tumor progression. They generally act by directly or indirectly inhibiting a broad array of immune functions, such as cytotoxic T-cell activation, pro-inflammatory cytokine (i.e., IL-1, IL-6, IFN-Y) secretion, antigen presentation and NK cell modulation.
Treg promoters: TGF-β, IL-10
Moreover, TGF-β and IL-10 are known Treg activators. Recently, various studies have reported elevated serum levels of these immunosuppressive regulators in NSCLC patients and proposed their potential prognostic utilization as tumor biomarkers. IL-10 levels were demonstrated to be overexpressed in NSCLC patient sera compared to healthy controls and associated with stage progression and inadequate response to treatment (29). A possible mode of action driving reduced survival in NSCLC patients overexpressing IL-10 has been linked to the IL-10 mediated suppression of pro-inflammatory cytokine IL-2 (30). Not surprisingly, IL-10 has been implicated as a potentially significant prognostic biomarker both individually and together with other serum tumor markers, including IL-6, MCP-1, CEA, CA125, ProGRP (31-33). Similarly, TGF-β has also been found at significantly high serum levels in both lung cancer patients and patients at risk for lung cancer (34,35). Its overexpression was correlated with progression, metastasis and poorer prognosis and thus was recommended as a possible biomarker for NSCLC oncogenesis (36). Aside from promoting Treg de-novo differentiation and suppressing cytotoxic T cell differentiation, TGF-β may act in concert with COX-2/PGE2 to convert naturally occurring immunostimulatory CD4+T cells into immunosuppressive CD4+CD25brightFOXP3+ T-regs in lung cancer patients. This may offer an explanation for both the high serum levels of TGF-β, COX-2, PGE2 and CD4+CD25brightFOXP3+ T-regs found in NSCLC and the inadequacy of T-reg depletion as a sufficient therapeutic strategy (37).
IL-17A
IL-17A is a unique pro-inflammatory cytokine due to its dual and paradoxical involvement in tumor management. It is secreted mainly by CD4+ Th17 cells, CD8+ T cells and macrophages and it acts by enhancing the cytotoxic T-cell mediated anti-tumor response and by stimulating vascular endothelial cell migration, angiogenesis and tumor progression (38). Significantly higher IL-17A serum levels have been widely reported in NSCLC patients and have been correlated with smoking status, increased lymph node invasion and metastatic disease (39,40). More specifically, high serum IL-17A levels in NSCLC is positively correlated with VEGF levels, suggesting a possible axis for promoting angiogenesis (41). Overall, several meta-analyses have concluded that high IL-17A serum levels lead to poorer prognosis in NSCLC patients (42-44).
IL-6: promoter of myeloid differentiation
Multiple studies have found IL-6 to be at elevated serum levels in advanced lung cancer, particularly in squamous cell and large cell carcinomas (45-47). Martín and colleagues found serum IL-6 levels over 130 to have a poor prognosis with a median survival of only 48 days (P=0.002) (46). Serum levels also trended higher with malnutrition and stage advancement in patients with more extensive disease. As a known acute phase reactant and promoter of myeloid differentiation, IL-6 has been observed to have a close relationship with other markers for the acute phase response such as ESR, CRP, ferritin, and a-1 antitrypsin (46). While its utility as a biomarker for survival in advanced stages is more understood, its role in early-stage cancers is understudied. Kaminska and colleagues found IL-6 to be an independent prognostic factor for only the initial 3 years of observation, but not for the complete follow-up period of 6 years (48).
Peripheral blood biomarkers
Prognostic nutritional index (PNI)
PNI, calculated from the serum albumin level and total lymphocyte count in a sample of peripheral blood, is a widely used index of patient nutritional and immunological status and was initially used to evaluate operative risk in gastrointestinal surgical patients (49). Subsequent literature has shown PNI to be a reliable predictor for both acute postoperative complications as well as survival outcomes in various solid tumors, particularly NSCLC (Table 1).
Table 1
| Role | Author | No. points | Stage (%) | Pathology (%) | End points | Outcome |
|---|---|---|---|---|---|---|
| PNI | Li et al., 2018 (50)* | 5,085 | I–IV | – | OS | Low PNI correlated with poor prognosis in early-stage NSCLC. Adenocarcinoma was less likely to have low PNI |
| Kos et al., 2015 (51) | 138 | I–III: 64 | Adeno: 57; SC: 53; other: 28 | OS | Low PNI (<49.5) is an independent predictor of poor prognosis in NSCLC patients | |
| Okada et al., 2017 (52) | 248 | I: 73; II: 15; III: 12 | Adeno: 76; SC: 16; other: 8 | OS | Low PNI correlated with postoperative complications and poor survival in resected NSCLC | |
| Qiu et al., 2015 (53) | 1,416 | I: 55; II: 21; III: 25 | Adeno: 53; SC: 40; other: 8 | OS | Low PNI (<52) was a significant prognostic factor in NSCLC | |
| Shoji et al., 2016 (54) | 141 | I: 100 | Adeno: 77; other: 23 | RFS | Low PNI group had a significantly shorter RFS in stage 1 NSCLC |
*meta-analysis. PNI, prognostic nutritional index; NSCLC, non-small cell lung cancer; OS, overall survival; Adeno, adenocarcinoma; SC, squamous cell carcinoma; RFS, recurrence-free survival.
Li and colleagues performed a meta-analysis of ten studies published between 2015–2017 including 5,085 patients (50). They found that a low PNI was highly correlated with poor prognosis in early-stage NSCLC patients (HR =1.96, 95% CI: 1.44–2.67, P=0.01). Most studies included in the analysis were from Asian populations. They also found tumor histology to be predictive of low PNI; patients with adenocarcinoma were significantly less likely to have low PNI (HR =0.59, 95% CI: 0.47–0.74). These findings generally agree with other meta-analyses showing that a low PNI is predictive of poor prognosis in colorectal and gastric cancers (55,56). In a retrospective study of 138 patients, Kos and colleagues found a PNI below 49.5 to predict poor prognosis in NSCLC, calculating a median OS of only 7.0 months in low PNI patients compared to 33.0 months in high PNI patients (P<0.001) (51). In addition, Okada and colleagues found low PNI to be correlated with postoperative complications (P=0.042) as well as poor survival (HR =2.18, 95% CI: 1.08–4.21, P=0.031) by multivariate analysis in their cohort of 248 patients with resected NSCLC (52). These findings agree with a study done by Qiu and colleagues who retrospectively studied 1416 patients undergoing radical surgery (53). They found a low PNI to be a significant prognostic factor in NSCLC; patients with a PNI below 52 had 1-, 3-, and 5-year survival rates of 80%, 61%, and 50%, respectively, compared to 85%, 72%, and 60% in patients with a PNI above 52 (P<0.001). Furthermore, Shoji and colleagues studied a cohort of 141 consecutive stage I NSCLC patients and found that a low PNI predicted recurrence (P<0.001) (54).
Neutrophil: lymphocyte ratio (NLR)
The NLR is known to be an indicator for systemic inflammatory responses which play an important role in the proliferation, angiogenesis, and progression of tumor cells (57). Under pathologic stress, there may be an increase in the number of neutrophils and a simultaneous decrease in lymphocytes, causing an increase in measured NLR. A neutrophilic response can have a negative immunomodulatory effect on cancer cells by suppressing cytotoxic T-cells and, likewise, a decreased lymphocyte response can aid in tumor invasion and progression due to impaired cell-mediated immunity (58).
The effects of NLR on prognosis have been widely studied in gastrointestinal cancers: higher NLR values correlate with worse prognosis (59). This relationship was also found to be true in breast (60), pancreatic (61), ovarian (62) and other cancers. Numerous studies have found a high NLR to be a reliable biomarker for poor prognosis in early NSCLC (Table 2).
Table 2
| Role | Author | No. points | Stage (%) | Pathology (%) | End points | Outcome |
|---|---|---|---|---|---|---|
| NLR | Kos et al., 2015 (51) | 138 | I–III: 64 | Adeno: 57; SC: 53; other: 28 | OS | Higher NLR (≥3.24) is an independent predictor of poor prognosis in NSCLC patients |
| Zhang et al., 2015 (63) | 678 | I: 45; II: 20; IIIA: 35 | Adeno: 41; SC: 47; other: 12 | DFS, OS | Preoperative NLR is significant prognostic indicator in primary operable NSCLC patients. High-risk patients based on NLR do not benefit from adjuvant therapy | |
| Shimizu et al., 2015 (64) | 334 | I: 72; II: 16; III: 12 | Adeno: 69; SC: 21; large cell: 7; other: 3 | OS | Elevated NLR >2.5 is a predictive factor for poor survival after complete resection of NSCLC | |
| Takahashi et al., 2015 (65) | 343 | I: 100 | Adeno:75; SC: 19; other: 6 | RFS, distant metastasis | Preoperative high NLR is a predictor of poor prognosis and associated with more frequent metastasis in completely resected stage 1 NSCLC | |
| Sarraf et al., 2009 (66) | 177 | I: 47; II: 25; III: 25 | Adeno: 49; SC: 32; large cell: 9; mixed: 3; other: 8 | OS | Increased NLR associated with higher stage, but also independent predictor of survival after complete resection and can stratify high death risk in stage 1 patients |
NLR, neutrophil: lymphocyte ratio; NSCLC, non-small cell lung cancer; OS, overall survival; Adeno, adenocarcinoma; SC, squamous cell carcinoma; DFS, disease-free survival; RFS, recurrence-free survival.
Kos and colleagues studied 88 patients with early NSCLC and found that patients with NLR above 3.24 had significantly worse OS (19 vs. 59 months, P<0.001) (51). Zhang and colleagues examined a much larger sample, 678 patients (45 stage I, 20 stage II and 35 stage IIIA), and found elevated NLR to independently predict both OS (HR =1.62, 95% CI: 1.30–2.02, P<0.001) and DFS (HR =1.59, 95% CI: 1.28–1.99, P<0.001) (63). Shimizu and colleagues also found elevated NLR >2.5 to be a predictive factor for poor survival after surgical resection (P<0.001) in their cohort of 334 patients (72 stage I, 16 stage II and 12 stage III) (64). Furthermore, Takahashi and colleagues studied 343 stage I NSCLC patients and found that an elevated NLR was associated with not only poor prognosis (P<0.001) but also conferred a higher likelihood of metastasis after complete resection (P<0.001) (65). In a multivariate analysis, they also showed that elevated NLR is an independent prognostic factor (HR =2.13, 95% CI: 1.31–3.52, P=0.003). Similarly, Sarraf and colleagues retrospectively examined 178 NSCLC patients after complete resection and found that an increase in pathologic stage was associated with elevated NLR (P=0.02) (66). After adjusting for stage, an elevated NLR independently predicted poor prognosis (HR =1.10, 95% CI: 1.03–1.17, P=0.004). While an independently elevated neutrophil count was not found to be correlated with poor prognosis (P=0.49), an elevated lymphocyte count had a protective effect on outcome (HR =0.62, P=0.01) likely due to the role of T cells in tumor suppression through cell-mediated immunity and cytokine production. NLR also has implications for patients treated with other modalities such as stereotactic radiation therapy. Cannon and colleagues found elevated pretreatment NLR to be significantly associated with poor survival in patients with early NSCLC undergoing SBRT therapy (P=0.005) (67).
Platelet: lymphocyte ratio (PLR)
Elevated platelets can be a sign of a systemic inflammatory response and previous research has found a high PLR to be associated with poor prognosis in gastric, breast, and colorectal cancers (68). It is thought that various proinflammatory cytokines released by platelets, such as platelet-derived growth factor (PDGF) and thrombospondin, play an important role in inflammation and tumor cell angiogenesis and progression (69). In a meta-analysis by Gu and colleagues that examined eleven studies and included 3,430 patients, seven of the studies focused on early-stage NSCLC. They found elevated PLR to be associated with both poor OS (HR =1.42, 95% CI: 1.25–1.61, P<0.001) and poor DFS (HR =1.19, 95% CI: 1.02–1.40, P=0.03) (68). Similarly, a study by Cannon and colleagues reported that high PLR was negatively associated with survival in early NSCLC being treated with stereotactic body radiation therapy (HR =4.0, 95% CI: 1.5–11.0, P=0.01) (67). While findings from Unal and colleagues agreed with the association between PLR and poor prognosis (69), their sample included mostly advanced stage NSCLC patients. PLR may also be a useful utility for diagnostic purposes as Kemal and colleagues have found PLR to be elevated in lung cancer patients when compared to healthy controls (PLR: 245.1 vs. 148.2, respectively, P=0.002) (70).
Some studies have failed to find PLR to be a reliable prognostic factor (Table 3).
Table 3
| Role | Author | No. points | Stage (%) | Pathology (%) | End points | Outcome |
|---|---|---|---|---|---|---|
| PLR | Gu et al., 2016 (68)* | 3,430 | I–IV | – | DFS, OS | Elevated PLR was associated with poor OS and DFS, particularly in Caucasians |
| Cannon et al., 2015 (67) | 59 | I: 100 | Adeno: 58; SC: 32; unspecified: 10 | OS, non-local failure | High NLR and high PLR are significant prognostic indicators for poor survival in stage 1 NSCLC patients treated with stereotactic radiation | |
| Unal et al., 2013 (69) | 94 | II: 10; IIIA: 43; IIIB: 48 | Adeno: 16; SC: 70; unclassified: 14 | DFS, OS | Pretreatment high PLR were associated with significantly shorter disease-free and overall survival rates | |
| Kemal et al., 2014 (70) | 81 | I–IIIA: 27 | Adeno: 40; SC: 33; other: 27 | PLR levels | PLR values were significantly higher in NSCLC patients compared to the healthy subjects (245.1 vs. 148.2) | |
| Pinato et al., 2014 (71) | 220 | IA: 34; IB: 23; IIA: 21; IIB: 9; IIIA: 13 | Adeno: 60; SC: 24; large cell: 1; mixed: 4; other: 11 | OS | Only a limited fraction of patients had an elevated PLR, with no significant impact on survival | |
| Zhang et al., 2015 (72) | 678 | I: 56; II: 9; III: 35 | Adeno: 41; SC: 47; other: 12 | DFS, OS | NLR was superior to PLR as a predictive factor for survival in primary operable NSCLC patients |
*meta-analysis. PLR, platelet: lymphocyte ratio; NSCLC, non-small cell lung cancer; DFS, disease-free survival; OS, overall survival; Adeno, adenocarcinoma; SC, squamous cell carcinoma.
A study by Pinato and colleagues prospectively examined 220 NSCLC patients undergoing surgical treatment and found no association between PLR and OS (P=0.32) (71); however, only 3% of patients in this study met cutoffs for elevated PLR, representing a relatively low sample. Similarly, Wu and colleagues found no significant survival difference in an analysis of 366 advanced NSCLC patients with elevated PLR and normal NLRs (OS: P=0.705, PFS: P=0.309) (73), leading to their speculation that PLR was better suited as a complementary, rather than independent, prognostic factor alongside NLR. Zhang and colleagues also found NLR to be a stronger predictor for survival when compared to PLR (72).
Platelet count
The primary function of platelets is to contribute to hemostasis, but these cells also play a role in the systemic inflammatory response. Jain and colleagues suggest three major mechanisms by which platelets promote tumorigenesis (74). First, platelets can aggregate and create a “cloak” around tumor cells protecting them from immune cell cytotoxic activity. Activated platelets express membrane protein P-selectin which leads to platelet aggregation and interaction with sulfoglycolipid ligands on tumor cells (75). Platelet membrane glycoprotein GP IIb–IIIa also has a role in binding to tumor cells in addition to other platelets (76). Second, platelets store and release various growth factors, proteases, and pro-inflammatory cytokines which help in tumor growth, invasion, and angiogenesis. These factors include VEGF, PDGF, fibroblast growth factor (FGF) and insulin-like growth factor (IGF). Third, platelets bind to tumor cells to make platelet-tumor cell emboli which subsequently extravasate to the metastatic niche. In vitro platelet activation by the tumor promotes release of microvesicles with platelet membrane proteins, increasing invasive potential through increased adhesion, proliferation, chemotaxis and survival of cancer cells (77).
When considering use of platelets as a cancer biomarker, thrombocytosis has been associated with poor prognosis and shorter survival in multiple types of solid tumors including breast, lung, colon, gastric and ovarian cancer (78). Furthermore, multiple studies have found high platelet count to be an independent predictor of poor prognosis in stage I NSCLC (Table 4).
Table 4
| Role | Author | No. points | Stage (%) | Pathology (%) | End points | Outcome |
|---|---|---|---|---|---|---|
| Platelet count | Sulibhavi et al., 2020 (12) | 103 | I: 100 | SC: 54; adeno: 32; large cell: 14 | RFS | High platelet count (>253 K) was independently associated with recurrence in pT1 NSCLC |
| Tomita et al., 2008 (79) | 250 | I: 64; II: 15; III–IV: 16 | Adeno: 69; other: 27 | OS | The 5-year survival of patients with and without thrombocytosis was 28.87% and 63.73%, respectively; thrombocytosis was independently prognostic | |
| Yu et al., 2013 (80) | 510 | I: 46; II: 25; III: 29 | Adeno: 50; SC: 46; other: 5 | DFS, OS | Thrombocytosis predicted poor survival and increased risk of disease progression | |
| Zhang et al., 2015 (81)* | 5,884 | – | Adeno: 37; SC: 42 | OS, TNM stage | Pooled meta-analysis results found high platelet to negatively predict OS and be associated with advanced TNM stage | |
| Lymphocyte count | Kobayashi et al., 2012 (10) | 237 | I: 86; II: 14 | Adeno: 67; SC: 22; large cell: 5; other: 5 | OS, recurrence | Low peripheral lymphocyte count was an indicator for poor prognosis and recurrence of NSCLC |
| Reuben et al., 2020 (11) | 236 | I–IV | – | T cell density | High T cell density in peripheral blood was associated with longer OS | |
| Sulibhavi et al., 2020 (12) | 103 | I: 100 | SC: 54; adeno: 32; large cell: 14 | RFS | High lymphocyte count (>1.8 K) was independently associated with recurrence in pT1 NSCLC |
*meta-analysis. NSCLC, non-small cell lung cancer; SC, squamous cell carcinoma; Adeno, adenocarcinoma; RFS, recurrence-free survival; OS, overall survival; DFS, disease-free survival.
Sulibhavi and colleagues examined 103 patients with stage I NSCLC and reported elevated platelet count to be associated with recurrence (5-year RFS of 72% in high platelet group vs. 92% in low platelet group, P=0.02) (12). Tomita and colleagues had similar findings in their study of 240 patients undergoing surgical resection (79). Although only 6% of their cohort had preoperative thrombocytosis, the 5-year survival in these patients was significantly lower compared to patients with normal platelet counts (29% vs. 64%, respectively, P<0.001). Yu and colleagues not only found thrombocytosis to predict poor OS (3-year survival of 75% with normal platelets vs. 59% with elevated platelets), but also increased risk of disease progression (HR =1.57, 95% CI: 1.01–2.45, P=0.03) (80). A meta-analysis performed by Zhang and colleagues looked at twelve studies of 5,884 patients and found a significant association with poor OS (HR =1.74, 95% CI: 1.39–2.19, P<0.001) (81). Furthermore, elevated platelets had an association with advanced TNM stage (OR =2.65, 95% CI: 1.77–3.97). While platelet function in tumorigenesis occurs primarily in the tumor microenvironment, peripheral platelet count is affected by systemic inflammation and may have clinical significance as a prognostic marker for early-stage NSCLC.
Conclusions
While the immune microenvironment surrounding the tumor has a significant role in modulating progression and ultimately prognosis, the immune response in the peripheral blood can aid in the development of inexpensive and non-invasive clinical markers. The interactions of immune cells, cytokines, and other peripheral immune markers may shift the immune response towards a conducive or inhibitory role in tumor defense (Table 5, Figure 1).
Table 5
| Role | Author | No. points | Stage (%) | Pathology (%) | End points | Outcome |
|---|---|---|---|---|---|---|
| T cells | Kobayashi et al., 2012 (10) | 237 | I: 86; II: 14 | Adeno: 67; SC: 22; large cell: 5; other: 5 | OS, recurrence | Low peripheral lymphocyte count was an indicator for poor prognosis and recurrence of NSCLC |
| Reuben et al., 2012 (11) | 236 | I–IV | – | T cell density | High T cell density in peripheral blood was associated with longer OS | |
| Sulibhavi et al., 2020 (12) | 103 | I: 100 | SC: 54; adeno: 32; large cell: 14 | RFS | High lymphocyte count (>1.8 K) was independently associated with recurrence in pT1 NSCLC | |
| T-regs | Li et al., 2009 (15) | 51 | I/II: 6; III: 20; IV: 25 | SC: 15; adeno: 29; SC/adeno: 4; large cell: 1; nondiff: 2 | Cell density, Treg activity | High Tregs count was found in peripheral blood of stage I NSCLC. Tregs proliferation and activity was suppressed in co-culture with activated CD4+ T cells |
| Chen et al., 2014 (16) | 49 | I: 18; II: 5; III: 14; IV: 12 | SC: 17; adeno: 26; SC/adeno: 2; large cell: 1; other: 3 | Cell density | High Tregs count was found in peripheral blood of NSCLC. Tregs count significantly increased as stage progressed | |
| NK cells | Picard et al., 2018 (18) | 176 | – | – | Cell density, NCR expression | CD56dim CD16+ NK cells are present at lower serum levels whereas CD56dim CD16– NK cells are at higher levels compared to healthy controls. NK cells exhibited a significant downregulation in the NCR NKp46 suggestive of lower cytotoxic potential |
| MDSCs | Yamauchi et al., 2018 (27) | 42 | I: 17; II: 12; III: 13 | SC: 16; adeno: 26 | Cell density | Both MDSC subtypes are increased in early-stage NSCLC. Mo-MDSCs, particularly, are more numerous in the peripheral blood and express higher levels of suppressive cytokines compared to those in tumor or nodal environment |
NSCLC, non-small cell lung cancer; NK, natural killer; MDSC, myeloid-derived suppressor cell; Adeno, adenocarcinoma; SC, squamous cell carcinoma; OS, overall survival; RFS, recurrence-free survival.
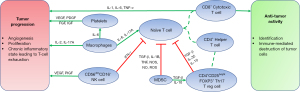
As well, peripheral markers may provide clinicians with easily accessible information on patient risk and prognosis. In NSCLC, both CD3+/CD4+ and CD8+ T lymphocytes are responsible for the identification and cell-mediated destruction of tumor cells. While previous research has shown high levels of T lymphocytes in the tumor microenvironment confers a longer survival, further research is needed to conclusively delineate the role of peripheral T lymphocytes. Nonetheless, current literature suggests that peripheral T lymphocyte cytokine signaling may augment activity of tumor T lymphocytes and serve as an important biomarker for prognosis. Several other peripheral immune cells, such as regulatory T lymphocytes and NK cells, have been implicated as potential biomarkers as well.
In addition, other biomarkers that can be easily measured from routine blood draw may hold important prognostic information. The PNI, NLR, PLR, and absolute platelet count have all been shown to be significantly correlated with prognosis in multivariate analyses and may be used for risk stratification in early NSCLC. Ongoing research investigating the role of microRNAs in the immunoregulation of NSCLC and its prognostic significance will provide another potential tool for clinicians to use. Further studies are needed to investigate their role in a large heterogeneous population with uniform histology and stage to definitively assess their interactions with clinical outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kei Suzuki) for the series “Immune Response in Lung Cancer” published in AME Medical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-20-122/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-122/coif). The series “Immune Response in Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carbone DP, Gandara DR, Antonia SJ, et al. Non-small-cell lung cancer: role of the immune system and potential for immunotherapy. J Thorac Oncol 2015;10:974-84. [Crossref] [PubMed]
- Crosbie PA, Shah R, Summers Y, et al. Prognostic and predictive biomarkers in early stage NSCLC: CTCs and serum/plasma markers. Transl Lung Cancer Res 2013;2:382-97. [PubMed]
- Church TR, Black WC, Aberle DR, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res 2019;11:943-53. [Crossref] [PubMed]
- Suzuki K, Kachala SS, Kadota K, et al. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res 2011;17:5247-56. [Crossref] [PubMed]
- Showe MK, Kossenkov AV, Showe LC. The peripheral immune response and lung cancer prognosis. Oncoimmunology 2012;1:1414-6. [Crossref] [PubMed]
- Tian C, Lu S, Fan Q, et al. Prognostic significance of tumor-infiltrating CD8+ or CD3+ T lymphocytes and interleukin-2 expression in radically resected non-small cell lung cancer. Chin Med J (Engl) 2015;128:105-10. [Crossref] [PubMed]
- De Vita F, Orditura M, Auriemma A, et al. Serum concentrations of proinflammatory cytokines in advanced non small cell lung cancer patients. J Exp Clin Cancer Res 1998;17:413-7. [PubMed]
- Kobayashi N, Usui S, Kikuchi S, et al. Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer 2012;75:223-7. [Crossref] [PubMed]
- Reuben A, Zhang J, Chiou SH, et al. Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nat Commun 2020;11:603. [Crossref] [PubMed]
- Sulibhavi A, Asokan S, Miller MI, et al. Peripheral blood lymphocytes and platelets are prognostic in surgical pT1 non-small cell lung cancer. Ann Thorac Surg 2020;109:337-42. [Crossref] [PubMed]
- Shimizu K, Nakata M, Hirami Y, et al. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol 2010;5:585-90. [Crossref] [PubMed]
- Okita R, Saeki T, Takashima S, et al. CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep 2005;14:1269-73. [Crossref] [PubMed]
- Li L, Chao QG, Ping LZ, et al. The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC. Cancer Biother Radiopharm 2009;24:357-67. [Crossref] [PubMed]
- Chen C, Chen D, Zhang Y, et al. Changes of CD4+CD25+FOXP3+ and CD8+CD28- regulatory T cells in non-small cell lung cancer patients undergoing surgery. Int Immunopharmacol 2014;18:255-61. [Crossref] [PubMed]
- Meloni F, Morosini M, Solari N, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28- T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol 2006;67:1-12. [Crossref] [PubMed]
- Picard E, Godet Y, Laheurte C, et al. Circulating NKp46+ natural killer cells have a potential regulatory property and predict distinct survival in non-small cell lung cancer. Oncoimmunology 2018;8:e1527498. [Crossref] [PubMed]
- Bruno A, Focaccetti C, Pagani A, et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia 2013;15:133-42. [Crossref] [PubMed]
- Tsartsalis D, Grapsa D, Skopeliti M, et al. In vitro exposure of NK-92 cells to serum from patients with non-small cell lung cancer impairs their cytotoxicity. Anticancer Res 2015;35:1543-8. [PubMed]
- Schütt P, Schütt B, Switala M, et al. Prognostic relevance of soluble human leukocyte antigen-G and total human leukocyte antigen class I molecules in lung cancer patients. Hum Immunol 2010;71:489-95. [Crossref] [PubMed]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162-74. [Crossref] [PubMed]
- de Goeje PL, Bezemer K, Heuvers ME, et al. Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer. Oncoimmunology 2015;4:e1014242. [Crossref] [PubMed]
- Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 2006;16:53-65. [Crossref] [PubMed]
- Chen S, Akbar SM, Abe M, et al. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol 2011;166:134-42. [Crossref] [PubMed]
- Liu CY, Wang YM, Wang CL, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14−/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol 2010;136:35-45. [Crossref] [PubMed]
- Yamauchi Y, Safi S, Blattner C, et al. Circulating and tumor myeloid-derived suppressor cells in resectable non-small cell lung cancer. Am J Respir Crit Care Med 2018;198:777-87. [Crossref] [PubMed]
- Pogoda K, Pyszniak M, Rybojad P, et al. Monocytic myeloid-derived suppressor cells as a potent suppressor of tumor immunity in non-small cell lung cancer. Oncol Lett 2016;12:4785-94. [Crossref] [PubMed]
- De Vita F, Orditura M, Galizia G, et al. Serum interleukin-10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest 2000;117:365-73. [Crossref] [PubMed]
- Neuner A, Schindel M, Wildenberg U, et al. Cytokine secretion: clinical relevance of immunosuppression in non-small cell lung cancer. Lung Cancer 2001;34:S79-82. [Crossref] [PubMed]
- Pan YW, Zhou ZG, Wang M, et al. Combination of IL-6, IL-10, and MCP-1 with traditional serum tumor markers in lung cancer diagnosis and prognosis. Genet Mol Res 2016; [Crossref] [PubMed]
- Daly S, Rinewalt D, Fhied C, et al. Development and validation of a plasma biomarker panel for discerning clinical significance of indeterminate pulmonary nodules. J Thorac Oncol 2013;8:31-6. [Crossref] [PubMed]
- Loosen SH, Schulze-Hagen M, Leyh C, et al. IL-6 and IL-8 serum levels predict tumor response and overall survival after TACE for primary and secondary hepatic malignancies. Int J Mol Sci 2018;19:1766. [Crossref] [PubMed]
- Takizawa H, Tanaka M, Takami K, et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 2001;163:1476-83. [Crossref] [PubMed]
- Barthelemy-Brichant N, David JL, Bosquée L, et al. Increased TGFbeta1 plasma level in patients with lung cancer: potential mechanisms. Eur J Clin Invest 2002;32:193-8. [Crossref] [PubMed]
- Huang AL, Liu SG, Qi WJ, et al. TGF-β1 protein expression in non-small cell lung cancers is correlated with prognosis. Asian Pac J Cancer Prev 2014;15:8143-47. [Crossref] [PubMed]
- Baratelli F, Lee JM, Hazra S, et al. PGE(2) contributes to TGF-beta induced T regulatory cell function in human non-small cell lung cancer. Am J Transl Res 2010;2:356-67. [PubMed]
- Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol 2009;183:4169-75. [Crossref] [PubMed]
- Chen X, Wan J, Liu J, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer 2010;69:348-54. [Crossref] [PubMed]
- Xu C, Hao K, Yu L, et al. Serum interleukin-17 as a diagnostic and prognostic marker for non-small cell lung cancer. Biomarkers 2014;19:287-90. [Crossref] [PubMed]
- Pan B, Che D, Cao J, et al. Interleukin-17 levels correlate with poor prognosis and vascular endothelial growth factor concentration in the serum of patients with non-small cell lung cancer. Biomarkers 2015;20:232-9. [Crossref] [PubMed]
- Wang XF, Zhu YT, Wang JJ, et al. The prognostic value of interleukin-17 in lung cancer: a systematic review with meta-analysis based on Chinese patients. PLoS One 2017;12:e0185168. [Crossref] [PubMed]
- Zeng Y, Zhang Q, Wang H, et al. Prognostic significance of interleukin-17 in solid tumors: a meta-analysis. Int J Clin Exp Med 2015;8:10515-36. [PubMed]
- Zhang X, Weng W, Xu W, et al. Prognostic significance of interleukin 17 in cancer: a meta-analysis. Int J Clin Exp Med 2014;7:3258-69. [PubMed]
- Songür N, Kuru B, Kalkan F, et al. Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori 2004;90:196-200. [Crossref] [PubMed]
- Martín F, Santolaria F, Batista N, et al. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine 1999;11:80-6. [Crossref] [PubMed]
- De Vita F, Orditura M, Auriemma A, et al. Serum levels of interleukin-6 as a prognostic factor in advanced non-small cell lung cancer. Oncol Rep 1998;5:649-52. [Crossref] [PubMed]
- Kaminska J, Kowalska M, Kotowicz B, et al. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis. M-CSF - an independent prognostic factor. Oncology 2006;70:115-25. [Crossref] [PubMed]
- Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg 1980;139:160-7. [Crossref] [PubMed]
- Li D, Yuan X, Liu J, et al. Prognostic value of prognostic nutritional index in lung cancer: a meta-analysis. J Thorac Dis 2018;10:5298-307. [Crossref] [PubMed]
- Kos FT, Hocazade C, Kos M, et al. Assessment of prognostic value of "neutrophil to lymphocyte ratio" and "prognostic nutritional index" as a sytemic inflammatory marker in non-small cell lung cancer. Asian Pac J Cancer Prev 2015;16:3997-4002. [Crossref] [PubMed]
- Okada S, Shimada J, Kato D, et al. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg 2017;104:296-302. [Crossref] [PubMed]
- Qiu C, Qu X, Shen H, et al. Evaluation of prognostic nutritional index in patients undergoing radical surgery with nonsmall cell lung cancer. Nutr Cancer 2015;67:741-7. [Crossref] [PubMed]
- Shoji F, Morodomi Y, Akamine T, et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer 2016;98:15-21. [Crossref] [PubMed]
- Yang Y, Gao P, Chen X, et al. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and a meta-analysis. Oncotarget 2016;7:58543-52. [Crossref] [PubMed]
- Yang Y, Gao P, Song Y, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol 2016;42:1176-82. [Crossref] [PubMed]
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5-14. [PubMed]
- Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. [Crossref] [PubMed]
- Mallappa S, Sinha A, Gupta S, et al. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis 2013;15:323-8. [Crossref] [PubMed]
- Faria SS, Fernandes PC Jr, Silva MJ, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience 2016;10:702. [PubMed]
- An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010;15:516-22. [Crossref] [PubMed]
- Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother 2009;58:15-23. [Crossref] [PubMed]
- Zhang H, Zhang L, Zhu K, et al. Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: based on a large cohort study. PLoS One 2015;10:e0126496. [Crossref] [PubMed]
- Shimizu K, Okita R, Saisho S, et al. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J Surg Oncol 2015;13:291. [Crossref] [PubMed]
- Takahashi Y, Horio H, Hato T, et al. Prognostic significance of preoperative neutrophil-lymphocyte ratios in patients with stage I non-small cell lung cancer after complete resection. Ann Surg Oncol 2015;22:S1324-31. [Crossref] [PubMed]
- Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425-8. [Crossref] [PubMed]
- Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol 2015;10:280-5. [Crossref] [PubMed]
- Gu X, Sun S, Gao XS, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Sci Rep 2016;6:23893. [Crossref] [PubMed]
- Unal D, Eroglu C, Kurtul N, et al. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev 2013;14:5237-42. [Crossref] [PubMed]
- Kemal Y, Yucel I, Ekiz K, et al. Elevated serum neutrophil to lymphocyte and platelet to lymphocyte ratios could be useful in lung cancer diagnosis. Asian Pac J Cancer Prev 2014;15:2651-4. [Crossref] [PubMed]
- Pinato DJ, Shiner RJ, Seckl MJ, et al. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer 2014;110:1930-5. [Crossref] [PubMed]
- Zhang H, Xia H, Zhang L, et al. Clinical significance of preoperative neutrophil-lymphocyte vs platelet-lymphocyte ratio in primary operable patients with non-small cell lung cancer. Am J Surg 2015;210:526-35. [Crossref] [PubMed]
- Wu G, Yao Y, Bai C, et al. Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non-small cell lung cancer patients. Thorac Cancer 2015;6:275-87. [Crossref] [PubMed]
- Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol 2010;30:2362-7. [Crossref] [PubMed]
- Garcia J, Callewaert N, Borsig L. P-selectin mediates metastatic progression through binding to sulfatides on tumor cells. Glycobiology 2007;17:185-96. [Crossref] [PubMed]
- Karpatkin S, Pearlstein E, Ambrogio C, et al. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest 1988;81:1012-9. [Crossref] [PubMed]
- Italiano JE Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol 2010;17:578-84. [Crossref] [PubMed]
- Buergy D, Wenz F, Groden C, et al. Tumor-platelet interaction in solid tumors. Int J Cancer 2012;130:2747-60. [Crossref] [PubMed]
- Tomita M, Shimizu T, Hara M, et al. Prognostic impact of thrombocytosis in resectable non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2008;7:613-5. [Crossref] [PubMed]
- Yu D, Liu B, Zhang L, et al. Platelet count predicts prognosis in operable non-small cell lung cancer. Exp Ther Med 2013;5:1351-4. [Crossref] [PubMed]
- Zhang X, Ran Y. Prognostic role of elevated platelet count in patients with lung cancer: a systematic review and meta-analysis. Int J Clin Exp Med 2015;8:5379-87. [PubMed]
Cite this article as: Asokan S, Cheung AY, Pavesi F, Bains A. Prognostic significance of peripheral blood immune response in early-stage non-small cell lung cancer: a narrative review. AME Med J 2021;6:38.

