
Recalcitrant posterior urethral stenoses: a narrative review of refractory bladder neck contractures and vesicourethral anastomotic stenoses after treatment for localized prostate cancer
Introduction
Stenosis of the posterior urethra can be a devastating complication for patients and challenging problem for clinicians to manage. Bladder neck contracture (BNC) specifically refers to stenosis of the proximal prostatic urethra/bladder neck, in which the prostate is still in situ, such as after transurethral resection of the prostate (TURP), photo-vaporization of the prostate (PVP), or after radiation for prostate cancer. In contrast, vesico-urethral anastomotic stenosis (VUAS) describes stenosis after radical prostatectomy (RP) or robotic assisted laparoscopic prostatectomy (RALP) of the sewn anastomosis of urethra to bladder (1). Posterior urethral stenosis (PUS) is typically a broader term used to described any pathologic stenoses of the bladder neck to the distal end of the membranous urethra (2). It is important that the correct nomenclature is used to promote communication, evaluation and management of these patients.
Prostate cancer is the most commonly diagnosed cancer in men in the United States, with approximately 1 in 9 men being diagnosed within their lifetime at an average age of 66 years old. However, most men will not die of prostate cancer and the 5-year survival rate is nearly 100% for localized prostate cancer, meaning most men will survive long enough to undergo at least one treatment modality (3). Patients who subsequently develop BNC or VUAS may suffer from recurring symptoms of dysuria, urinary frequency, urinary incontinence, and urinary retention. When refractory, such issues can result in a poor quality of life.
The purpose of this review is to define the frequency of BNC and VUAS in relation to different treatment modalities for prostate cancer, examine the different treatment options, understand their effectiveness, and to develop a treatment algorithm for managing these complicated patients. We present the following article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-20-191/rc).
Methods
A literature review was performed to evaluate the incidence, etiology, diagnosis and management of BNC and VUAS after treatment for localized prostate cancer. BNC may occur after treatment for benign prostatic obstruction; however, this is an additional heterogeneous group of patients and procedures was not the focus of this review. A PubMed search was performed using the search terms “bladder neck contracture”, “vesicourethral anastomotic stenosis”, “posterior urethral stenosis”, and “urethral stricture”, from the years 2000–2020.
Discussion
Incidence and etiology
RP
There are several risk factors that can result in poor healing at the site of the surgical anastomosis and lead to the development of VUAS after RP. For simplification, these can be divided into three categories: pre-operative, intraoperative and post-operative. Pre-operative risk factors include cigarette smoking and significant coronary artery disease (4). Intraoperative risk factors include high estimated blood loss (EBL), excessive luminal narrowing, local tissue ischemia, failed mucosal apposition, anastomotic tension, and limited surgeon experience (5). Post-operative risk factors include prolonged urine leak, pelvic hematoma, surgical clip migration and adjuvant external beam radiation therapy (EBRT) (6,7). VUAS related to RP typically occurs within the first six months after surgery, with almost none occurring more than 24 months after surgery (8).
The reported incidence of VUAS after RP is variable, with studies showing a rate of 1–8.4% (5,8-11). With the transition from an open to a robotic approach, and increasing robotic surgeon experience, the incidence of VUAS in recent reports is now lower. In a prospective study published in 2019, comparing open radical prostatectomy (ORP) with RALP in 4,003 men, they found an incidence of VUAS of 1.3% after 24 months of follow-up in the RALP group. The risk was 2.2 times higher in the ORP group (RR =2.21, 95% CI: 1.38–3.53) (9). This is similar to other studies looking to the incidence of BNC after RALP (5,10,12). The lower incidence of VUAS in the RALP group may be due to improved visualization, lower blood loss, better mucosal inversion, and improved instrument maneuverability within the deep pelvis (10). The exception to this are those patients undergoing salvage prostatectomy, as these patients are at increased risk for ischemia and have a reported to have a incidence of VUAS of 22–40% (13,14). Additionally, adjuvant EBRT increases the risk of VUAS, with reported incidences of 3–8% (15,16). These different incidences are summarized in Table 1.
Table 1
| Treatment | Stenosis incidence (%) | Literature review | Comments |
|---|---|---|---|
| RP (ORP and RALP) | 1.9–8.4 | (5,8-12) | Incidence of VUAS was <1% in last 500 cases (all ORP) (5); all BNC were in ORP group (12) |
| RALP | 0–1.4 | (9,10,12) | |
| RP + EBRT | 2.7–4.7 | (5,8,16) | |
| Salvage RP | 22–40 | (13,14) | |
| EBRT | 1–13 | (2,8) | Highly variable on duration of follow-up: <7% in <5 years, 10–18% in 5–10 years |
| BT | 1.8–15 | (2,8,17) | Lower incidence (0–5%) with low dose BT (2); 15% stricture incidence with high dose BT (17) |
| EBRT + BT | 5.2–32 | (8,17-19) | Outlier study, 32% stricture incidence with a high fractional dosing (17); otherwise incidence <8% |
| Cryotherapy | 1.9–2.5 | (8,20) | |
| HIFU | 3.6–4.8 | (2,21) |
RP, radical prostatectomy, ORP, open radical prostatectomy, RALP, robotic assisted laparoscopic prostatectomy, EBRT, external beam radiation therapy, BT, brachytherapy, HIFU, high intensity focused ultrasound.
Radiation therapy
Radiation as primary therapy for localized prostate cancer can be delivered as EBRT, brachytherapy (BT), or a combination of both. Ionizing radiation causes direct cell/tissue injury through the creation of reactive oxygen species (ROS), which leads to oxidative damage and ultimately cell death (22). Radiation also causes indirect cell damage to the surrounding tissues by promoting gene expression of pro-inflammatory cytokines, leading to inflammation and ultimately fibrosis and reduced vascularity (22). This also accounts for the delayed presentation of radiation induced stenosis, which presents at a mean of 20–22 months after treatment and with increasing incidence over longer term follow-up (2,8).
The incidence of stenosis after radiation is variable across the literature depending on the delivery method, dosage and fractionation. In the CaPSURE study, radiation strictures were less frequent than VUAS (1–5% vs. 8%), but rates increase progressively over time (8). Combined BT and EBRT combined have the highest stricture rates. Hindson et al. found a stricture rate of 3–32% two years following EBRT and high-dose BT that was variable based on fractionation of dosing (17). Other studies report a stricture rate of 4–9% following combination therapy (18,19,23).
Cryotherapy and high intensity focused ultrasound (HIFU)
Cryotherapy involves local ablation of prostatic tissues by using extremely low temperatures created by argon and helium gas delivered through targeted needles. The rapid freezing and slow cooling produces a thermal shock that results in cell necrosis and apoptosis by coagulation necrosis (20). There is urethral sloughing of necrotic tissue, which may result in acute urinary obstruction, but there is also localized ischemic changes resulting in inflammation and may ultimate lead to fibrosis and urethral stenosis. Modern cryotherapy uses urethral warming to prevent urethral damage and now the reported incidence of urethral stenosis is generally <3% (2,20).
HIFU uses targeted thermo-ablation delivered through a transrectal probe. It causes direct cellular destruction by heating the tissue and resulting in coagulative necrosis. Like cryotherapy, there is sloughing of the necrotic tissue that can frequently result in initial urinary obstruction, sometimes requiring endoscopic intervention, approximately 25% of the time. Later follow-up shows BNC post-HIFU from 3.6–4.8% (2,21,24). Table 1 illustrates the incidence of urethral stenosis after primary treatment of prostate cancer as seen in the literature.
Diagnosis and evaluation
Patients presenting for evaluation of VUAS/BNC will often present with obstructive lower urinary tract symptoms (LUTS) and/or incontinence (stress, urgency or both) and a history of treatment for prostate cancer. In more severe cases of stenosis, urinary retention and need for temporary supravesical diversion, such as a suprapubic tube (SPT), may be present. There is not a validated workup for LUTS after treatment of prostate cancer, however, it is important to focus on elements in the history related to timing and treatment modality for prostate cancer, complications related to treatment, such as urine leak, post-op hematoma, or administration adjuvant radiation therapy or any salvage therapies. Prior treatments for VUAS/BNC also need to be considered. For instance, in patients that have been previously dilated, continence status afterwards and/or time to stenosis recurrence, should be factored into the decision-making process.
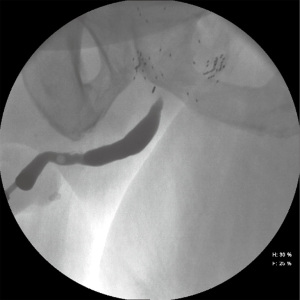
Laboratory tests should include urinalysis and culture, basic metabolic panel, and a recent PSA to determine cancer status. The workup should include the standard evaluations of bladder outlet obstruction—namely uroflow, post-void residual (PVR), and cystourethroscopy. Office cystourethroscopy allows for evaluation of the anterior urethra, tissue integrity, length/location/caliber of the stenosis, and any complicating factors such as stones, eroded surgical clips, or radiation changes. A pediatric cystoscope can be very useful for patients with narrow (>8 Fr) but not obliterative stenoses. If the stenosis is >8 Fr, the bladder can also be evaluated for capacity, severe detrusor overactivity and radiation cystitis. In patients whom the stenosis cannot be adequately visualized on cystourethroscopy, retrograde urethrogram +/– voiding cystourethrogram (RUG/VCUG) should be performed. RUG by itself is a poor imaging modality for visualizing the posterior urethra (Figure 1), but can be combined with VCUG to determine the exact location and length of the stenosis.
Before proceeding to surgery, the patient’s life expectancy, cancer status, and the psychological component of post-op urinary incontinence and ability/willingness to possibly undergo multiple procedures needs to be considered. Shared decision making should be employed when determining a surgical plan. It is important to factor in the most important goals of surgery (decreased urinary frequency, improve urinary incontinence, ability to void normally, rapid recovery, etc.) before intervention.
Treatment
Management of PUS, BNC and VUAS after primary prostate cancer treatment has multiple treatment options, varying in complexity and invasiveness. Minimally invasive treatment options include intermittent self-dilation (ISD), endoscopic incision of the bladder neck, or placement of a Wall stent. Open reconstruction should be considered for longer or recurrent strictures but requires a highly motivated patient as these procedures are complex, potentially morbid and often require delayed placement of an artificial urinary sphincter (AUS). It is also important to consider urinary diversion in the treatment algorithm for these patients, as it is sometimes the best option.
Endoscopic management
For stenoses that are short (<2 cm), it is reasonable to consider initial endoscopic management (25). Dilation is only successful in approximately 50% of patients after one treatment, but may increase to 97% with multiple treatment attempts, with a de novo incontinence rate of 0.6 percent (26). ISD may also be initiated after dilation or urethrotomy, to prevent recurrence in patients who are motivated and have good dexterity with (27,28). However, there is no consensus on schedule or duration of ISD. The regimen used at our institution is ISD with a 16Fr catheter once daily for one month, every other day for one month, and then weekly for one month.
Urethrotomy or incision of the bladder neck can be performed with a cold knife, hot knife, laser, or resection loop depending on surgeon preference. Radial incisions should be made at least at two sites to release the cicatrix, but the 6 o’clock position should be avoided due to proximity of the rectum and increased risk for rectal injury in these patients (29). Eventual success can often be achieved, but typically involves at least one, and sometimes multiple repeat procedures. For a standard transurethral incision of the bladder neck (TUIBN) with a hot or cold knife, the average initial success rate is 74% (range, 17–87%) with an eventual success rate of 97% (range, 88–100%) and a de novo incontinence rate of 4.6% (range, 0–12.5%) (11,26,30-34). For patients with recurrent or intractable stenosis, a “deep” TUIBN can be performed. This is typically a two-stage procedure with an initial aggressive incision, to the level of the periprostatic fat, resulting in planned severe incontinence, followed by delayed placement of anAUS). After a deep TUIBN, Ramirez et al. noted 78% de novo incontinence, of whom 67% underwent AUS placement 3 months later (35). Overall, the initial success rate for “deep” TUIBN is 90.5% (range, 71–100%), with an eventual success rate of 86–100% and de novo incontinence of 78–100% with repeat TUIBN (26,31,35,36).
Recently, Warner, reports a novel endoscopic technique of TUIBN with transverse mucosal reapproximation (37). In this procedure, TUIBN is performed with a hot knife with standard longitudinal incisions made at 3 and 9 o’clock. A short rigid ureteroscope and a laparoscopic suturing device are then used to reapproximate the mucosa transversely, in a Heineke-Milkulicz fashion. Outcomes are promising, with no stricture recurrence in 12 of 13 patients (92%) and an average follow-up of 6 months (range, 3–10 months), however, more patients and longer follow-up is needed. They also do not report on de novo incontinence.
Injection of the bladder neck at time of TUIBN with a biologic modifier, such at mitomycin C (MMC), steroids, or other agents has been widely performed for recalcitrant BNCs/VUAS. MMC, an antiproliferative agent, is the most widely studied and it is typically injected at a concentration of 0.3–0.4 mg/mL. Success of MMC for BNC after one procedure is reported to be 58–75% after one procedure and 75–89% after two procedures (30,38-40). Variable adverse events are reported. However, up to a 7% develop severe complications, such as osteitis pubis and rectourethral fistula formation (39). Steroid injection, specifically triamcinolone, at time of bladder neck incision, is hypothesized to work by decreasing scar formation by increasing collagenase production. Studies have shown that steroids can delay the time to recurrence (8 vs. 3.5 months), but not the overall rate of recurrence (41,42). Hyaluronic acid (HA) and carboxymethylcellulose (CMC) injection may also delay time to recurrence, but longer term follow-up is needed (43).
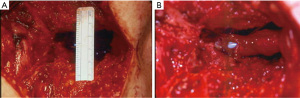
Placement of a bladder neck stent is another option for endoscopic management of the refractory BNC. This is typically reserved for patients who have failed multiple prior incisions/dilations and who are not good surgical candidates or not interested in an open repair. Like the “deep” TUIBN, this is typically a two-stage procedure with the intention of making the patient incontinent with subsequent placement of an AUS. The UroLume stent (American Medical Systems, Minnetonka, MN) is no longer commercially available and was often complicated by tissue regrowth, calcification and/or urethral stenosis (2). However, some still believe that it is a reasonable option for severe strictures in patients unwilling or unable to undergo surgical repair (44-48). Off-label use of vascular wall stents can be used in a similar fashion to the UroLume (Figure 2). At our institution, we use the Epic vascular self-expanding stent system (Boston Scientific, Marlborough, MA) for this purpose. Overall, stents for BNC have an initial success of 65.2% (range, 50–89%), with an eventual success of 86.1% (range, 76–100%), yet a de novo incontinence rate of 100% (26).
Endoscopic treatment of BNC/VUAS is a reasonable option for many patients. Initial success rates are moderate, but eventual success rates are high with repeat endoscopic procedures or adjuvant treatment, such as ISD. It is important to recognize risks of recurrent stenosis and de novo incontinence and to counsel patients appropriately as to the likelihood of needing multiple procedures if an endoscopic management pathway is chosen. It is also reasonable to progress to more aggressive surgical treatment if initial endoscopic treatment fails.
Open repair
Open VUAS repair is potentially morbid surgery that requires a highly motivated and well-informed patient. It is challenging for the surgeon and requires the whole surgical armamentarium, like the progressive steps for a posterior urethroplasty. The repair may be performed via a transperineal (Figure 3), abdominal or abdomino-perineal (AP) approach (Figure 4). If an AP technique is used, a two-team approach is preferred. The general goals are the same as that for a posterior urethroplasty: excision of all scar tissue, spatulate the urethra and perform a tension free anastomosis with mucosa to mucosa apposition. One should be prepared to perform maneuvers to shorten the distance between the distal and proximal ends of the urethra, such as corporal splitting and a partial or total pubectomy. VCUG/RUG should be performed postoperatively and show an open urethra. After recovery, these patients typically require a staged AUS that is performed 6–12 months later if the bladder neck remains open. This is due to the fact that the external sphincter is typically excised or damaged in order to get to the bladder neck during a transperineal approach. Shahrour et al. report a transperineal technique where a buccal mucosal graft (BMG) is placed dorsally after dissection of the urethra under the pubic bone. This limits risk of rectal injury and need for extensive urethral dissection, pubectomy and corporal splitting, but incontinence rates in their small series was 100% with plans for staged AUS placement (49). Transvesical ventral BMG inlay cystoplasty has also been described for open BNC repair in a small case series with reasonable success, but follow-up is limited and continence rates are not reported (50). The reported success of VUAS reconstruction is variable and the data is limited to just case series. Song et al. reported an initial success rate of 74% in the literature, with an eventual success rate of 96% (26). More recent studies have reported a success rate of 92% in 12 patients with an average follow-up of 74 months and 80% in 20 patients with two years of follow-up (51,52). This success rate increased to 90% after a single endoscopic intervention and an additional 24 months of follow-up (52). They also compared continence rates using a perineal versus an abdominal approach and found a much higher rate of incontinence in the perineal group (100% vs. 10%). They attributed this finding to trans-sphincteric mobilization of the urethra during a transperineal approach (52). Patients that fail open VUAS repair are usually salvaged by a SPT or more invasive supravesical diversion.

The preoperative continence and location of stenosis should thus be taken into account when determining a surgical approach. Those with pre-operative continence and a stenosis proximal to the perineal membrane likely benefit from a robotic or non-perineal approach as this can avoid dissection of the external sphincter and limit the need for staged AUS. Additionally, those that do develop incontinence may have better AUS durability and ease of placement due to lack of prior perineal dissection (53). AUS complications are not infrequent in this population due to compromised urethras. Urethral risk factors include history of pelvic radiation, recalcitrant BNC, urethral reconstruction, prior AUS explantation for infection or erosion, and/or prior (or current) urethral stent (54,55). Brant et al. have shown that explantation rates increase with the number of urethral risk factors present, and there is a 25% explantation rate when 3 risk factors are present compared with 2.6% when there are 0 (54). Mock et al. looked at a population with transcorporal AUS cuffs and found the probability of cuff erosion in patients with 2 or more urethral risk factors was 1.65 times the probability of erosion in those with 0 or 1 urethral risk factor (55). Thus, the need for staged AUS placement should not be taken lightly.
Robotic-assisted laparoscopic (RAL) repair
RAL repairs are becoming increasingly more common for treatment of nonradiated, recalcitrant BNC/VUAS. The most widely reported is the RAL V-Y plasty. Standard port configuration and positioning for pelvic surgery is utilized. The bladder neck is approached by recreating the space of Retzius by dropping the bladder off the abdominal wall and mobilizing the urethra under the pubic bone. The bladder is entered anteriorly with a V-shaped incision and the long arm of the Y is a longitudinal incision through the scar tissue. The apex of the V is then advanced to the distal aspect of the longitudinal incision, a Y-V advancement bladder flap. Pre-operative and intraoperative cystoscopy can be helpful in defining the anatomic location of the stenosis, the external sphincter, and where to make the incision (56). Additional RAL techniques involve circumferential freeing of the bladder neck and excision of scar tissue in patients after prior prostatectomy, or excision of scar tissue and partial prostatectomy in those where the prostate is in situ. This can be done with an anterior or posterior approach (56).
Three studies to date have reported reasonable success with robotic-assisted laparoscopic V-Y plasty for patients with both recalcitrant BNC after benign endoscopic procedures as well as VUAS after RP. Of 31 total patients, the overall success rate was 81% at 11 months follow-up (56-58). Five of these patients had a V-Y plasty performed after a RP, and 3 of 5 were a success at a mean follow-up of 13 months. None of these patients had SUI post-operatively. One patient had a severe post-op complication with rhabdomyolysis, as well as an intersymphyseal fistula ultimately requiring a pubectomy and rectus flap (56). RAL V-Y plasty may be a viable treatment option especially when considering its improved continence rates over the perineal approach.
Additional RAL techniques have been described utilizing BMGs. Avallone et al. report a RAL reconstruction of a BNC with subtrigonal inlay of a BMG. They utilize a space of Retzius sparing, transvesical approach via transabdominal robotic access. A V-shaped incision is made through ventral aspect of the BNC with a Y-limb extending into the prostatic urethra. The fibrotic scar tissue is excised and a triangular-shaped BMG is sewn into the defect (59). Zhao reports repair of a recalcitrant VUAS using the da Vinci SP (Intuitive Surgical, Sunnyvale, CA) with transvesical access through a suprapubic incision, avoiding all abdominal dissection. Incisions were made into the stenotic area at 12, 3 and 9 o’clock and a BMG was placed dorsally for augmentation (53). Larger studies and longer follow-up are needed to evaluate the outcomes of these techniques.
Urinary diversion
Ultimately, some patients with recalcitrant VUAS/BNC may most benefit from urinary diversion. This option should be considered for patients who have received adjuvant radiation, have very long or obliterative strictures, have failed multiple prior endoscopic or open procedures, and/or had multiple AUS erosions. The status of the bladder should also be considered, as patients with end stage bladders (minimal capacity, radiation cystitis, severe bladder spasms/pain) would also likely benefit from a urinary diversion rather than urethral/bladder neck reconstruction. Additionally, depending on the selected urinary diversion, it may be less morbid with a shorter operative time than bladder neck/urethral reconstructive surgery, so it should be considered for patients with poor performance status or aversion to multiple major surgeries.
There are multiple options for urinary diversion, and these can be divided into incontinent and continent diversions. Incontinent diversions include SPT, ileal conduit, transverse colon conduit, or cutaneous ureterostomy. Continent diversions include creation of a catheterizable stoma with or without an augmentation cystoplasty, or a continent catheterizable neobladder, such as an Indiana pouch. Several patient factors need to be considered when choosing a supravesical diversion. These include, anatomical factors (bladder capacity, presence of radiation cystitis, urinary fistulae, body habitus, prior abdominal surgeries, prior bowel resections, and body habitus), as well as medical comorbidities, cancer status, functional status and renal function. Prior to creation of a catheterizable stoma, hand dexterity, independence and motivation of the patient need to also be evaluated.
Patients undergoing supravesical urinary diversions (i.e., urinary conduits or catheterizable pouches), the defunctionalized bladder needs to be considered. Reported complications related to a retained bladder include hemorrhage, pain/spasms, pyocystis, and neoplastic transformation (60,61). Literature is conflicting regarding the need for concomitant cystectomy in the setting of supravesical diversion performed for benign indications, as most of these complications are quite rare and can often be managed conservatively (62). However, these patients in particular often have bladder outlet obstruction and/or a history of radiation, both of which are known risk factors for retained bladder complications, with up to 71% of these patients developing complications from a defunctionalized bladder and up to 43% requiring surgery or a secondary cystectomy (62-64). Additionally, it has been shown that simple cystectomy can typically be performed quickly, with minimal complications and blood loss, which is even more reason that strong consideration should be given to concomitant cystectomy at time of supravesical diversion in these patients (65,66).
There are several different options for catheterizable stomas. Small bowel (Monti) or appendix (Mitroffanoff) with tunneling through the detrusor muscle and creation of a flap-valve mechanism (Mitroffanoff principle) can be used (Figure 5) (67). A Monti is a good option when the appendix is not present or suitable and the native bladder is to be used for the tunnel. If possible, it is best to tunnel the channel into the inferior aspect of the native bladder, as this helps prevent stasis and the resulting pooling of mucus, stones and recurrent urinary tract infections. Additional length can be gained by performing a spiral Monti (Figure 6). However, this technique is often complicated by difficulty catheterizing and need for subfascial surgical revisions on long-term follow-up (68,69). Alternatively, the ileocecal valve can be used as a continent mechanism for the catheterizable stoma, as is done for an Indiana pouch or for a cecal augment and ileal catheterizable stoma (70).
Stoma location and associated complications are not an uncommon occurrence. It is very useful to work closely with a stoma nurse to preoperatively determine placement of the stoma. It is important to consider the patient’s body habitus, belt line, folds of skin, and hand dexterity. The stoma may be placed in the umbilicus or the abdominal wall. However, there is a higher incidence of stenosis with stomas in the umbilicus, so this has fallen out of favor (71). The primary stomal complications are stomal stenosis and incontinence. The incidence of stomal stenosis is 13–55% overall, but this rate is closer to the lower range, of this incidence when stomal construction with VQZ and V flaps is performed (71-75). Furthermore, this complication is usually relatively easy to treat with dilation or a skin-level surgical repair (71). Continence is achieved in in approximately 57–92% of patients (71,73,74). Redshaw et al. compared stomal complications between tunneled channels and cutaneous ileal cecocystoplasty and found a lower continence rate with tunneled channels (57% vs. 71%) and a higher rate of secondary procedures (50% vs. 13%) (74).
Uretero-enteric (UE) anastomosis techniques can be divided into refluxing (direct) versus nonreluxing (tunnelled) as well as individual (Bricker) versus conjoined (Wallace) techniques. Several tunnelled techniques have been described (Le Duc, Goodwin, Leadbetter), but these have largely fallen out of favor due to increased rates of anastomotic stricture and no significant difference in rates of pyelonephritis, stones, or azotemia (76-79). In the Bricker technique, the ureters are spatulated and anastomosed independently in an end-to side, freely refluxing fashion to the bowel segment. In the Wallace technique, the ureters are spatulated and then conjoined in either a head to tail or side by side fashion and then anastomosed to the proximal end of the ileal segment. In a relatively recent meta-analysis by Davis et al., there was no statistically difference in anastomotic stenosis rates between the two techniques, independent of confounding variables such as gender and radiotherapy, suggesting that technique should be based on surgeon preference (80).
UE strictures are a significant complication following urinary diversion, resulting in significant morbidity and often requiring further invasive intervention. In the radical cystectomy (RC) literature, the incidence of benign UE strictures is 1.3–12.7% with the Bricker anastomosis technique and is thought to be due to tissue ischemia with resulting scarring (81,82). With advancements in technology, intravenously administered indocyanine green (ICG) (Akorn Inc., Lake Forest, IL) with near-infrared fluorescence can be used for intraoperative imaging to assess ureter vascularity and determine where to best excise the distal ureter. For patients undergoing RC with ileal conduit and a Bricker anastomosis, studies have consistently found a decreased rate of UE stricture utilizing the ICG technology with a stricture rate of 0–3.2% in the ICG groups versus 6.6–16.7% in the non-ICG groups with a median follow-up of 12–23 months (81,83,84). Larger studies and longer follow-up is needed, but this technology shows promising results and may be particularly helpful in high-risk patients, such as those with history of radiation therapy.
Conclusions
Recalcitrant PUS, is a morbid complication that can occur after treatment for localized prostate cancer. BNC/VUAS occurs at different incidences depending on the primary treatment modality. In general, there are higher rates associated with radiation and long-term follow-up. However, the highest risk is after salvage prostatectomy. It is important that clinicians and physicians are aware of this complication and factor it into their decision making and counseling for prostate cancer treatment.
BNC/VUAS is a challenging condition to manage both for patients and clinicians. Patients can have bothersome symptoms of dysuria, urinary frequency, recurrent urinary tract infections, incontinence and urinary retention. Clinicians can become overwhelmed managing these patients as the decision making is complex and patients frequently require multiple interventions and long-term follow-up. It is critical to thoroughly evaluate these patients pre-operatively and to include them in decision making and to individualize a treatment plan.
In general, it is best to take a graded approach to treatment such that each successive intervention is more invasive. With more invasive treatments, there is often more de novo SUI and need for staged AUS. Certain procedures, such as deep TUIBN and transperineal VUAS repair assume severe post-operative incontinence and need for a staged AUS 6–12 months later. These open reconstructions are often technically challenging and require an able, educated and motivated patient. RAL techniques show promise with reasonable outcomes and lower rates of urinary incontinence in select patients. It is important to consider urinary diversion in patients with obliterative and refractory BNC/VUAS or in those who are unable or unwilling to undergo major surgery. Unfortunately, the literature for treatment of BNC/VUAS is poorly detailed, retrospective and overly heterogenous and there is a major need for a randomized controlled trial.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Lucas Wiegand) for the series “Radiation Urologic Reconstruction” published in AME Medical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-20-191/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-20-191/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-191/coif). The series “Radiation Urologic Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Latini JM, McAninch JW, Brandes SB, et al. SIU/ICUD Consultation On Urethral Strictures: Epidemiology, etiology, anatomy, and nomenclature of urethral stenoses, strictures, and pelvic fracture urethral disruption injuries. Urology 2014;83:S1-7. [Crossref] [PubMed]
- Herschorn S, Elliott S, Coburn M, et al. SIU/ICUD Consultation on Urethral Strictures: Posterior urethral stenosis after treatment of prostate cancer. Urology 2014;83:S59-70. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Borboroglu PG, Sands JP, Roberts JL, et al. Risk factors for vesicourethral anastomotic stricture after radical prostatectomy. Urology 2000;56:96-100. [Crossref] [PubMed]
- Erickson BA, Meeks JJ, Roehl KA, et al. Bladder neck contracture after retropubic radical prostatectomy: incidence and risk factors from a large single-surgeon experience. BJU Int 2009;104:1615-9. [Crossref] [PubMed]
- Hu JC, Gold KF, Pashos CL, et al. Role of surgeon volume in radical prostatectomy outcomes. J Clin Oncol 2003;21:401-5. [Crossref] [PubMed]
- Cormio L, Massenio P, Lucarelli G, et al. Hem-o-lok clip: a neglected cause of severe bladder neck contracture and consequent urinary incontinence after robot-assisted laparoscopic radical prostatectomy. BMC Urol 2014;14:21. [Crossref] [PubMed]
- Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol 2007;178:529-34; discussion 34. [Crossref] [PubMed]
- Modig KK, Godtman RA, Bjartell A, et al. Vesicourethral Anastomotic Stenosis After Open or Robot-assisted Laparoscopic Retropubic Prostatectomy-Results from the Laparoscopic Prostatectomy Robot Open Trial. Eur Urol Focus 2021;7:317-24. [Crossref] [PubMed]
- Breyer BN, Davis CB, Cowan JE, et al. Incidence of bladder neck contracture after robot-assisted laparoscopic and open radical prostatectomy. BJU Int 2010;106:1734-8. [Crossref] [PubMed]
- Giannarini G, Manassero F, Mogorovich A, et al. Cold-knife incision of anastomotic strictures after radical retropubic prostatectomy with bladder neck preservation: efficacy and impact on urinary continence status. Eur Urol 2008;54:647-56. [Crossref] [PubMed]
- Webb DR, Sethi K, Gee K. An analysis of the causes of bladder neck contracture after open and robot-assisted laparoscopic radical prostatectomy. BJU Int 2009;103:957-63. [Crossref] [PubMed]
- Corcoran NM, Godoy G, Studd RC, et al. Salvage prostatectomy post-definitive radiation therapy: The Vancouver experience. Can Urol Assoc J 2013;7:87-92. [Crossref] [PubMed]
- Ward JF, Sebo TJ, Blute ML, et al. Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J Urol 2005;173:1156-60. [Crossref] [PubMed]
- Daly T, Hickey BE, Lehman M, et al. Adjuvant radiotherapy following radical prostatectomy for prostate cancer. Cochrane Database Syst Rev 2011;CD007234. [Crossref] [PubMed]
- Macdonald OK, Lee RJ, Snow G, et al. Prostate-specific antigen control with low-dose adjuvant radiotherapy for high-risk prostate cancer. Urology 2007;69:295-9. [Crossref] [PubMed]
- Hindson BR, Millar JL, Matheson B. Urethral strictures following high-dose-rate brachytherapy for prostate cancer: analysis of risk factors. Brachytherapy 2013;12:50-5. [Crossref] [PubMed]
- Sullivan L, Williams SG, Tai KH, et al. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol 2009;91:232-6. [Crossref] [PubMed]
- Phan TP, Syed AMN, Puthawala A, et al. High Dose Rate Brachytherapy as a Boost for the Treatment of Localized Prostate Cancer. J Urol 2007;177:123-7. [Crossref] [PubMed]
- Rodríguez SA, Arias Fúnez F, Bueno Bravo C, et al. Cryotherapy for primary treatment of prostate cancer: intermediate term results of a prospective study from a single institution. Prostate Cancer 2014;2014:571576. [Crossref] [PubMed]
- Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol 2003;76:590-9. [Crossref] [PubMed]
- Kim JH, Jenrow KA, Brown SL. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J 2014;32:103-15. [Crossref] [PubMed]
- Aström L, Pedersen D, Mercke C, et al. Long-term outcome of high dose rate brachytherapy in radiotherapy of localised prostate cancer. Radiother Oncol 2005;74:157-61. [Crossref] [PubMed]
- Kahokehr AA, Peterson AC, Lentz AC. Posterior urethral stenosis after prostate cancer treatment: contemporary options for definitive management. Transl Androl Urol 2018;7:580-92. [Crossref] [PubMed]
- Wessells H, Angermeier KW, Elliott S, et al. Male Urethral Stricture: American Urological Association Guideline. J Urol 2017;197:182-90. [Crossref] [PubMed]
- Song J, Eswara J, Brandes SB. Postprostatectomy Anastomosis Stenosis: A Systematic Review. Urology 2015;86:211-8. [Crossref] [PubMed]
- Ivaz SL, Veeratterapillay R, Jackson MJ, et al. Intermittent self-dilatation for urethral stricture disease in males: A systematic review and meta-analysis. Neurourol Urodyn 2016;35:759-63. [Crossref] [PubMed]
- Kjaergaard B, Walter S, Bartholin J, et al. Prevention of urethral stricture recurrence using clean intermittent self-catheterization. Br J Urol 1994;73:692-5. [Crossref] [PubMed]
- Browne BM, Vanni AJ. Management of Urethral Stricture and Bladder Neck Contracture Following Primary and Salvage Treatment of Prostate Cancer. Curr Urol Rep 2017;18:76. [Crossref] [PubMed]
- Vanni AJ, Zinman LN, Buckley JC. Radial urethrotomy and intralesional mitomycin C for the management of recurrent bladder neck contractures. J Urol 2011;186:156-60. [Crossref] [PubMed]
- Anger JT, Raj GV, Delvecchio FC, et al. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol 2005;173:1143-6. [Crossref] [PubMed]
- Kravchick S, Lobik L, Peled R, et al. Transrectal ultrasonography-guided injection of long-acting steroids in the treatment of recurrent/resistant anastomotic stenosis after radical prostatectomy. J Endourol 2013;27:875-9. [Crossref] [PubMed]
- Eltahawy E, Gur U, Virasoro R, et al. Management of recurrent anastomotic stenosis following radical prostatectomy using holmium laser and steroid injection. BJU Int 2008;102:796-8. [Crossref] [PubMed]
- Yurkanin JP, Dalkin BL, Cui H. Evaluation of cold knife urethrotomy for the treatment of anastomotic stricture after radical retropubic prostatectomy. J Urol 2001;165:1545-8. [Crossref] [PubMed]
- Ramirez D, Zhao LC, Bagrodia A, et al. Deep lateral transurethral incisions for recurrent bladder neck contracture: promising 5-year experience using a standardized approach. Urology 2013;82:1430-5. [Crossref] [PubMed]
- Gousse AE, Tunuguntla HS, Leboeuf L. Two-stage management of severe postprostatectomy bladder neck contracture associated with stress incontinence. Urology 2005;65:316-9. [Crossref] [PubMed]
- Warner JN. Transurethral incision with transverse mucosal realignment for repair of posterior urethral strictures, technique and early outcomes. Urology Video Journal 2020;7:100044. [Crossref]
- Lyon TD, Ayyash OM, Ferroni MC, et al. Bipolar Transurethral Incision of Bladder Neck Stenoses with Mitomycin C Injection. Adv Urol 2015;2015:758536. [Crossref] [PubMed]
- Redshaw JD, Broghammer JA, Smith TG 3rd, et al. Intralesional injection of mitomycin C at transurethral incision of bladder neck contracture may offer limited benefit: TURNS Study Group. J Urol 2015;193:587-92. [Crossref] [PubMed]
- Farrell MR, Sherer BA, Levine LA. Visual Internal Urethrotomy With Intralesional Mitomycin C and Short-term Clean Intermittent Catheterization for the Management of Recurrent Urethral Strictures and Bladder Neck Contractures. Urology 2015;85:1494-9. [Crossref] [PubMed]
- Mazdak H, Izadpanahi MH, Ghalamkari A, et al. Internal urethrotomy and intraurethral submucosal injection of triamcinolone in short bulbar urethral strictures. Int Urol Nephrol 2010;42:565-8. [Crossref] [PubMed]
- Tavakkoli Tabassi K, Yarmohamadi A, Mohammadi S. Triamcinolone injection following internal urethrotomy for treatment of urethral stricture. Urol J 2011;8:132-6. [PubMed]
- Chung JH, Kang DH, Choi HY, et al. The effects of hyaluronic acid and carboxymethylcellulose in preventing recurrence of urethral stricture after endoscopic internal urethrotomy: a multicenter, randomized controlled, single-blinded study. J Endourol 2013;27:756-62. [Crossref] [PubMed]
- Erickson BA, McAninch JW, et al. Management for prostate cancer treatment related posterior urethral and bladder neck stenosis with stents. J Urol 2011;185:198-203. [Crossref] [PubMed]
- McNamara ER, Webster GD, Peterson AC. The UroLume stent revisited: the Duke experience. Urology 2013;82:933-6. [Crossref] [PubMed]
- Elliott DS, Boone TB. Combined stent and artificial urinary sphincter for management of severe recurrent bladder neck contracture and stress incontinence after prostatectomy: a long-term evaluation. J Urol 2001;165:413-5. [Crossref] [PubMed]
- Magera JS Jr, Inman BA, Elliott DS. Outcome analysis of urethral wall stent insertion with artificial urinary sphincter placement for severe recurrent bladder neck contracture following radical prostatectomy. J Urol 2009;181:1236-41. [Crossref] [PubMed]
- Eisenberg ML, Elliott SP, McAninch JW. Preservation of lower urinary tract function in posterior urethral stenosis: selection of appropriate patients for urethral stents. J Urol 2007;178:2456-60; discussion 2460-1. [Crossref] [PubMed]
- Shahrour W, Hodhod A, Kotb A, et al. Dorsal Buccal Mucosal Graft Urethroplasty for Vesico-Urethral Anastomotic Stricture Postradical Prostatectomy. Urology 2019;130:210. [Crossref] [PubMed]
- Da Silva RD, Marks JM, Kim FJ, et al. MP24-19 transvesical ventral buccal mucosa graft inlay cystoplasty for reconstruction of refractory bladder neck contractures after benign prostatic hyperplasia surgery: surgical technique and preliminary results. J Urol 2017;197:e309.
- Nikolavsky D, Blakely SA, Hadley DA, et al. Open reconstruction of recurrent vesicourethral anastomotic stricture after radical prostatectomy. Int Urol Nephrol 2014;46:2147-52. [Crossref] [PubMed]
- Giúdice CR, Lodi PE, Olivares AM, et al. Safety and effectiveness evaluation of open reanastomosis for obliterative or recalcitrant anastomotic stricture after radical retropubic prostatectomy. Int Braz J Urol 2019;45:253-61. [Crossref] [PubMed]
- Zhao LC. Management of Urethral Stenosis After Treatment for Prostate Cancer: NYU Case of the Month, August 2020. Rev Urol 2020;22:133-4. [PubMed]
- Brant WO, Erickson BA, Elliott SP, et al. Risk factors for erosion of artificial urinary sphincters: a multicenter prospective study. Urology 2014;84:934-8. [Crossref] [PubMed]
- Mock S, Dmochowski RR, Brown ET, et al. The Impact of Urethral Risk Factors on Transcorporeal Artificial Urinary Sphincter Erosion Rates and Device Survival. J Urol 2015;194:1692-6. [Crossref] [PubMed]
- Kirshenbaum EJ, Zhao LC, Myers JB, et al. Patency and Incontinence Rates After Robotic Bladder Neck Reconstruction for Vesicourethral Anastomotic Stenosis and Recalcitrant Bladder Neck Contractures: The Trauma and Urologic Reconstructive Network of Surgeons Experience. Urology 2018;118:227-33. [Crossref] [PubMed]
- Musch M, Hohenhorst JL, Vogel A, et al. Robot-assisted laparoscopic Y-V plasty in 12 patients with refractory bladder neck contracture. J Robot Surg 2018;12:139-45. [Crossref] [PubMed]
- Granieri MA, Weinberg AC, Sun JY, et al. Robotic Y-V Plasty for Recalcitrant Bladder Neck Contracture. Urology 2018;117:163-5. [Crossref] [PubMed]
- Avallone MA, Quach A, Warncke J, et al. Robotic-assisted Laparoscopic Subtrigonal Inlay of Buccal Mucosal Graft for Treatment of Refractory Bladder Neck Contracture. Urology 2019;130:209. [Crossref] [PubMed]
- Adeyoju AB, Lynch TH, Thornhill JA. The defunctionalized bladder. Int Urogynecol J Pelvic Floor Dysfunct 1998;9:48-51. [Crossref] [PubMed]
- Fazili T, Bhat TR, Masood S, et al. Fate of the leftover bladder after supravesical urinary diversion for benign disease. J Urol 2006;176:620-1. [Crossref] [PubMed]
- Lawrence A, Hu B, Lee O, et al. Pyocystis after urinary diversion for incontinence--is a concomitant cystectomy necessary? Urology 2013;82:1161-5. [Crossref] [PubMed]
- Engel RM. Complications of Bilateral Uretero-Ileo Cutaneous Urinary Diversion: A Review of 208 Cases. J Urol 1969;101:508-12. [Crossref] [PubMed]
- Eigner EB, Freiha FS. The Fate of the Remaining Bladder following Supravesical Diversion. J Urol 1990;144:31-3. [Crossref] [PubMed]
- Neulander EZ, Rivera I, Eisenbrown N, et al. Simple cystectomy in patients requiring urinary diversion. J Urol 2000;164:1169-72. [Crossref] [PubMed]
- Rowley MW, Clemens JQ, Latini JM, et al. Simple Cystectomy: Outcomes of a New Operative Technique. Urology 2011;78:942-5. [Crossref] [PubMed]
- Kaefer M, Retik AB. The Mitrofanoff principle in continent urinary reconstruction. Urol Clin North Am 1997;24:795-811. [Crossref] [PubMed]
- Leslie JA, Cain MP, Kaefer M, et al. A comparison of the Monti and Casale (spiral Monti) procedures. J Urol 2007;178:1623-7; discussion 1627. [Crossref] [PubMed]
- Whittam BM, Szymanski KM, Flack C, et al. A comparison of the Monti and spiral Monti procedures: A long-term analysis. J Pediatr Urol 2015;11:134.e1-6. [Crossref] [PubMed]
- Shen JK, Chan KG, Warner JN. Continent cutaneous ileocecal cystoplasty in the treatment of refractory bladder neck contracture and urinary incontinence after prostate cancer treatment. Can J Urol 2020;27:10093-8. [PubMed]
- De Ganck J, Everaert K, Van Laecke E, et al. A high easy-to-treat complication rate is the price for a continent stoma. BJU Int 2002;90:240-3. [Crossref] [PubMed]
- Itesako T, Nara K, Matsui F, Matsumoto F, et al. Clinical experience of the VQZ plasty for catheterizable urinary stomas. J Pediatr Urol 2011;7:433-7. [Crossref] [PubMed]
- Gowda BD, Agrawal V, Harrison SC. The continent, catheterizable abdominal conduit in adult urological practice. BJU Int 2008;102:1688-92. [Crossref] [PubMed]
- Redshaw JD, Elliott SP, Rosenstein DI, et al. Procedures needed to maintain functionality of adult continent catheterizable channels: a comparison of continent cutaneous ileal cecocystoplasty with tunneled catheterizable channels. J Urol 2014;192:821-6. [Crossref] [PubMed]
- Landau EH, Gofrit ON, Cipele H, et al. Superiority of the VQZ over the tubularized skin flap and the umbilicus for continent abdominal stoma in children. J Urol 2008;180:1761-5; discussion 1765-6. [Crossref] [PubMed]
- Pantuck AJ, Han KR, Perrotti M, et al. Ureteroenteric anastomosis in continent urinary diversion: long-term results and complications of direct versus nonrefluxing techniques. J Urol 2000;163:450-5. [Crossref] [PubMed]
- Hassan AA, Elgamal SA, Sabaa MA, et al. Evaluation of direct versus non-refluxing technique and functional results in orthotopic Y-ileal neobladder after 12 years of follow up. Int J Urol 2007;14:300-4. [Crossref] [PubMed]
- Shigemura K, Yamanaka N, Imanishi O, et al. Wallace direct versus anti-reflux Le Duc ureteroileal anastomosis: comparative analysis in modified Studer orthotopic neobladder reconstruction. Int J Urol 2012;19:49-53. [Crossref] [PubMed]
- Hohenfellner R, Black P, Leissner J, et al. Refluxing ureterointestinal anastomosis for continent cutaneous urinary diversion. J Urol 2002;168:1013-6; discussion 1016-7. [Crossref] [PubMed]
- Davis NF, Burke JP, McDermott T, et al. Bricker versus Wallace anastomosis: A meta-analysis of ureteroenteric stricture rates after ileal conduit urinary diversion. Can Urol Assoc J 2015;9:E284-90. [Crossref] [PubMed]
- Ahmadi N, Ashrafi AN, Hartman N, et al. Use of indocyanine green to minimise uretero-enteric strictures after robotic radical cystectomy. BJU Int 2019;124:302-7. [Crossref] [PubMed]
- Large MC, Cohn JA, Kiriluk KJ, et al. The impact of running versus interrupted anastomosis on ureterointestinal stricture rate after radical cystectomy. J Urol 2013;190:923-7. [Crossref] [PubMed]
- Doshi CP, Wozniak A, Quek ML. Near-infrared Fluorescence Imaging of Ureters With Intravenous Indocyanine Green During Radical Cystectomy to Prevent Ureteroenteric Anastomotic Strictures. Urology 2020;144:220-4. [Crossref] [PubMed]
- Shen JK, Jamnagerwalla J, Yuh BE, et al. Real-time indocyanine green angiography with the SPY fluorescence imaging platform decreases benign ureteroenteric strictures in urinary diversions performed during radical cystectomy. Ther Adv Urol 2019;11:1756287219839631. [Crossref] [PubMed]
Cite this article as: Blum R, Brandes S. Recalcitrant posterior urethral stenoses: a narrative review of refractory bladder neck contractures and vesicourethral anastomotic stenoses after treatment for localized prostate cancer. AME Med J 2022;7:6.


