
A review of the role of prostatic artery embolization in the management of post-radiation prostatitis
Introduction
Background
Definitive radiotherapy (RT) remains an important option for the treatment of localized prostate cancer (PCa). Brachytherapy (BT), stereotactic body radiation therapy (SBRT) and external beam radiation therapy (EBRT) represent several possible delivery mechanisms for RT. Patient demographics along with PCa characteristics and prostate size influence the decision for these treatment modalities. However, acute and chronic genitourinary (GU) toxicity after RT can occur in up to 33% of men (1) and can be exceedingly challenging to manage. Moreover, men with larger prostates suffer from a higher rate of chronic GU toxicity and chronic symptoms have been shown to occur at higher rates in patients receiving a high central urethral dose (2). Pinpointing prostatitis as the etiology of GU toxicity after RT is also challenging to do; as such radiation-induced prostatitis is poorly understood and not well-represented in the literature. Therefore, the exact incidence of prostatitis after RT has not been well reported and its management is poorly understood.
The National Institutes of Health categorizes prostatitis into four entities based on acuity and etiology (3). Types I and II prostatitis are acute and chronic, respectively, both of which are a result of bacterial infections. Types III and IV are non-bacterial and are classified according to the presence or absence of clinical symptoms, respectively. The prevalence of chronic prostatitis is between 1.8–8.2% (3) with symptoms including urinary frequency, urgency, dysuria, hesitancy, incontinence, sexual dysfunction and pain with ejaculation (4). Type III, also known as Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS), represents 90% of all cases of prostatitis (3,5). While the etiology is unclear, it is hypothesized that an inciting agent may cause inflammation or neurologic damage in or around the prostate, which leads to pelvic floor neuromuscular and/or neuropathic pain, with lower urinary tract symptoms (LUTS) representing a predisposing factor (6).
Radiation-induced prostatitis can be classified as Type III CP/CPPS and management can be approached as such. The treatment algorithm for these men therefore focuses on the “3 As” of chronic prostatitis: clearing any underlying infection with Anti-biotics, limiting reactive inflammation and associated symptoms with Anti-inflammatories, and alleviating LUTS with Alpha blockers (6,7). However, clinical efficacy of medical management of post-radiation prostatitis is variable (7,8) with up to 50% of men demonstrating no improvement of symptoms after medical therapy. We therefore describe here a brief review of the role of prostatic artery embolization (PAE) in the management of chronic GU toxicity/chronic prostatitis after definitive RT for PCa as an emerging therapy.
Objectives
- Understand the incidence of prostatitis after definitive RT for PCa;
- Know the medical management of prostatitis after definitive RT;
- Learn about the role of PAE for the management of medically recalcitrant radiation-induced prostatitis.
Methods
This is a brief review evaluating the role of PAE for the management of radiation-induced prostatitis.
PAE is a minimally invasive procedure that has been well studied for the improvement of LUTS in the setting of benign prostatic hyperplasia (BPH) (9,10). The technique is performed by an experienced interventional radiologist and involves transfemoral or transradial arterial access with catheterization of bilateral internal iliac arteries and subsequent selective micro-catheterization of bilateral prostatic arteries. Arterial embolization of bilateral prostatic arteries is then performed using a dilute mixture of gelatin microspheres (typically ~300 microns in diameter) with contrast and normal saline (Figure 1).
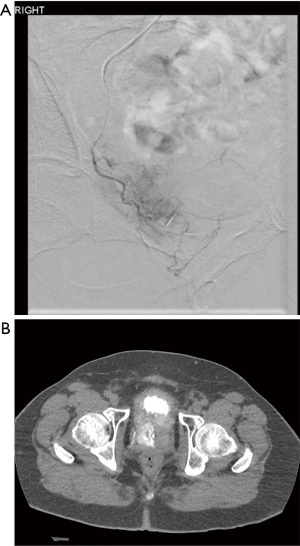
The procedure is typically performed in an outpatient setting. Patients are seen prior to the procedure by both the referring urologist as well as the interventional radiologist and are followed by the interventional radiologist at 6 weeks, 12 weeks, 6 months and annually after the procedure.
Adverse events after the procedure can be described as either “side effects” or “complications”. A “side effect” represents an expected adverse event, while a “complication” represents an unexpected adverse event that is related to treatment. Side effects occur in up to 50% of men and are a result of central gland acute ischemia (11). Urinary tract infection, spasms, frequency and pelvic achiness are the most common symptoms, but are mitigated by a standing “cocktail” of post-procedure medications including NSAIDs, antibiotics, urinary analgesics, and anti-spasmodic agents (Tables 1,2) (11). Complications occur as a result of non-target embolization of shared arterial vessels supplying pelvic organs and occur in less than 1–5% of patients. They include focal ischemic changes of the urinary bladder, penis and rectum, and resolve within 4–6 weeks with conservative management (12). PAE does NOT affect erectile function (12).
Table 1
| Side effect | Organs involved | Symptoms | Incidence |
|---|---|---|---|
| Urinary spasms/urgency | Bladder/prostate | Urinary spasms/frequency | 42% |
| Prostatitis/urethritis | Prostate/urethra | Burning and retropubic pain | 1.9–17% |
| Hematospermia | Seminal vesicles | Hematospermia | 16% |
| Hematuria | Bladder/prostate | Hematuria | 12% |
| Post-embolization syndrome | Prostate | Pain, nausea, fever | 10% |
| Urinary retention | Bladder/prostate | No urine output | 4.6% |
| Urinary tract infection | Bladder/prostate | Burning and retropubic pain | 2.5–4.6% |
PAE, prostatic artery embolization.
Table 2
| Scheduled |
| Ibuprofen 800 mg TID PRN × 7 days |
| Solifenacin 5 mg daily × 7 days |
| Phenazopyridine 100 mg TID × 7 days |
| Ciprofloxacin 500 mg BID × 7 days |
| PRN for severe symptoms |
| Methylprednisolone DosePak (4 mg × 21 tablets): |
| • Day 1: 8 mg before breakfast; 4 mg after lunch; 4 mg after dinner; 8 mg at bedtime |
| • Day 2: 4 mg before breakfast; 4 mg after lunch; 4 mg after dinner; 8 mg at bedtime |
| • Day 3: 4 mg before breakfast; 4 mg after lunch; 4 mg after dinner; 4 mg at bedtime |
| • Day 4: 4 mg before breakfast; 4 mg after lunch; 4 mg at bedtime |
| • Day 5: 4 mg before breakfast; 4 mg at bedtime |
| • Day 6: 4 mg before breakfast |
| Bisocodyl 20 mg daily × 7 days |
PAE effectiveness is measured quantitatively by evaluating urinary peak flow rate (Qmax) and prostate volume decrease (13) (Figure 2) and qualitatively by changes in patient reported outcomes, including International Prostate Symptom Score (IPSS), AUA Score, Quality of Life Questionnaire (QoL) and International Index of Erectile Function (IIEF) score. Significant Qmax improvement (100% improvement in some studies) and PV reduction (from 30–40%) (10) can be expected with concordant significant IPSS/AUA and QoL improvement, in the appropriate patient population.

The best candidates for PAE are men with at least moderate LUTS (AUA/IPSS ≥15) and “large” glands (size ≥60 cc3) without any prostate-related intervention. If men necessitate catheterization of any sort for urination, indwelling Foley catheter is the preference prior to PAE. It is more difficult to wean men off clean intermittent catheterization (CIC) after PAE than off an indwelling Foley catheter. If men have concurrent PCa, the best results are seen with men who have non-obstructive cancer that is centered in the peripheral zone. For men with lower urinary symptoms and concurrent PCa, PAE prior to definitive RT is the preference. For men who have radiation-induced prostatitis, PAE should ideally be reserved for men who are refractory to medical therapy, and at least 3 months time should be given for healing after completion of RT. Up to 30% of men will have recurrence of symptoms by 5 years, but those who responded well in the first place can be expected to respond again to PAE (14).
Discussion
GU toxicity after RT is a challenging diagnosis to manage. Isolating prostatitis as the primary etiology for GU toxicity after RT presents a diagnostic and management challenge. Radiation-induced prostatitis can be considered a form of CP/CPPS and medical therapy is the mainstay for these men but up to 50% are refractory (6). Evidence is significantly lacking for both the identification and management of this difficult diagnosis. While there have been several attempts at finding other therapies for these patients, such as transurethral prostatic injection of onabotulinumtoxin A and transurethral microwave thermotherapy, none have demonstrated any large-scale clinical benefit (15).
Although PAE has been shown to successfully improve LUTS in men with BPH, PAE for symptomatic improvement in men with chronic prostatitis after definitive RT—considered a type of late grade 2 or greater GU toxicity—has been only minimally evaluated. Our group has shown that PAE is safe and successful (up to 90%) in improving clinical symptoms and quality of life (80%) in men who have chronic prostatitis as a result of RT for PCa as reported by LUTS and chronic prostatitis scoring index (CPSI). There is also evidence to suggest that patients who require catheterization—either Foley or clean intermittent catheterization (CIC)—may able to be taken off successfully after PAE (16).
While PAE has largely been known to be effective for “large” glands (>60 gm), PAE has also been shown to be effective in men who have radiation-induced prostatitis with normal sized glands (16). This suggests that BPH was not the only contributing factor to chronic symptoms in these patients and thus necrosis induced from embolization may be synergistic along with volume reduction. It also suggests a reasoning for why men who do not have symptoms prior to radiation, may develop symptoms afterwards, even in the setting of a normal-sized gland.
Our study was the only one that has analyzed the efficacy of PAE for the management of medically refractory radiation-induced prostatitis (16), and this was only evaluating nine patients. Therefore, more studies will need to be performed to fully assess the role of PAE in the management of radiation-induced prostatitis.
Conclusions
Radiation-induced prostatitis is a challenging diagnosis for patients and their physicians. Medical therapy is often unsuccessful, and no other therapies or interventions have demonstrated consistent, large-scale benefit in refractory cases. PAE is a novel therapy for men with medically recalcitrant radiation-induced prostatitis that has been shown to be safe and clinically effective. Therefore, men with medically recalcitrant LUTS after definitive radiation for PCa should be considered for PAE.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Lucas Wiegand) for the series “Radiation Urologic Reconstruction” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-189/coif). The series “Radiation Urologic Reconstruction” was commissioned by the editorial office without any funding or sponsorship. NP reports personal fees from Canon Medical Systems, personal fees from Boston Scientific, Corp, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ohri N, Dicker AP, Showalter TN. Late toxicity rates following definitive radiotherapy for prostate cancer. Can J Urol 2012;19:6373-80. [PubMed]
- Hsu IC, Hunt D, Straube W, et al. Dosimetric analysis of radiation therapy oncology group 0321: the importance of urethral dose. Pract Radiat Oncol 2014;4:27-34. [Crossref] [PubMed]
- Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA 1999;282:236-7. [Crossref] [PubMed]
- Holt JD, Garrett WA, McCurry TK, et al. Common Questions About Chronic Prostatitis. Am Fam Physician 2016;93:290-6. [PubMed]
- de la Rosette JJ, Hubregtse MR, Meuleman EJ, et al. Diagnosis and treatment of 409 patients with prostatitis syndromes. Urology 1993;41:301-7. [Crossref] [PubMed]
- Anothaisintawee T, Attia J, Nickel JC, et al. Management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis. JAMA 2011;305:78-86. [Crossref] [PubMed]
- Nickel JC. Prostatitis. In: Kursh ED, Ulchaker JC. editors. Office Urology. Current Clinical Urology. Totowa: Humana Press, 2001.
- Cohen JM, Fagin AP, Hariton E, et al. Therapeutic intervention for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a systematic review and meta-analysis. PLoS One 2012;7:e41941. [Crossref] [PubMed]
- McWilliams JP, Bilhim TA, Carnevale FC, et al. Society of Interventional Radiology Multisociety Consensus Position Statement on Prostatic Artery Embolization for Treatment of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: From the Society of Interventional Radiology, the Cardiovascular and Interventional Radiological Society of Europe, Société Française de Radiologie, and the British Society of Interventional Radiology: Endorsed by the Asia Pacific Society of Cardiovascular and Interventional Radiology, Canadian Association for Interventional Radiology, Chinese College of Interventionalists, Interventional Radiology Society of Australasia, Japanese Society of Interventional Radiology, and Korean Society of Interventional Radiology. J Vasc Interv Radiol 2019;30:627-37.e1. [Crossref] [PubMed]
- Ray AF, Powell J, Speakman MJ, et al. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int 2018;122:270-82. [Crossref] [PubMed]
- Parikh N, Acharya V, Bhatia S. Prostate Artery Embolization - Adverse Events and Peri-Procedural Management. Tech Vasc Interv Radiol 2020;23:100692. [Crossref] [PubMed]
- Moreira AM, de Assis AM, Carnevale FC, et al. A Review of Adverse Events Related to Prostatic Artery Embolization for Treatment of Bladder Outlet Obstruction Due to BPH. Cardiovasc Intervent Radiol 2017;40:1490-500. [Crossref] [PubMed]
- de Assis AM, Moreira AM, de Paula Rodrigues VC, et al. Prostatic artery embolization for treatment of benign prostatic hyperplasia in patients with prostates > 90 g: a prospective single-center study. J Vasc Interv Radiol 2015;26:87-93. [Crossref] [PubMed]
- Costa NV, Torres D, Pisco J, et al. Repeat Prostatic Artery Embolization for Patients with Benign Prostatic Hyperplasia. J Vasc Interv Radiol 2020;31:1272-80. [Crossref] [PubMed]
- Smith CP. Male chronic pelvic pain: An update. Indian J Urol 2016;32:34-9. [Crossref] [PubMed]
- Parikh N, Keshishian E, Sharma A, et al. Prostatic Artery Embolization Is Safe and Effective for Medically Recalcitrant Radiation-Induced Prostatitis. Adv Radiat Oncol 2020;5:905-9. [Crossref] [PubMed]
Cite this article as: Parikh N, Manley B, Pow-Sang J, Yamoah K. A review of the role of prostatic artery embolization in the management of post-radiation prostatitis. AME Med J 2022;7:5.

