
Comparison between RECIST 1.1, Choi and PERCIST 1.0 criteria to evaluate response to SBRT of liver metastasis
Introduction
Improvements in tumor imaging, radiation therapy planning, delivery and motion management have contributed to the development of stereotactic body radiation therapy (SBRT) as a precise external beam radiation therapy used to deliver a high dose of radiation in a small number of fractions to extracranial lesions, minimizing the volume of surrounding normal tissues exposed to high dose levels (1).
SBRT is an effective and safe option for patients with oligometastatic disease, with very high rates of local control and promising estimations of disease-free survival (DFS) after SBRT, with scant toxicity (2-4). It is an alternative of local ablative therapy to treat oligometastatic inoperable patients (5) and liver primary tumors which are not candidate for surgery (NCCN Clinical Practice Guidelines in Oncology) (6).
Currently there are no guidelines for follow-up and assessment of response in tumors undergoing hepatic SBRT (7,8). Most published papers assess response of liver metastasis to SBRT with RECIST criteria, despite many authors have demonstrated that sometimes RECIST criteria underestimate the benefit of new therapies (9-12). Moreover, RECIST 1.1 criteria specify that “tumour lesions situated in a previously irradiated area, or in an area subjected to other loco-regional therapy, are usually not considered measurable by RECIST” (13).
Some authors have suggested new criteria to assess response to hepatic SBRT (14) or have proposed the use of PET-CT to better evaluate response (15,16).
In our study we analyzed imaging characteristics (size, attenuation coefficient and, if available, 18-FDG uptake) of liver metastasis treaded by SBRT and we compared the accuracy of different imaging criteria (RECIST 1.1, Choi and PERCIST 1.0) to assess treatment response. We did not use modified RECIST (mRECIST) criteria as we did not include in our series patients with hepatocellular carcinoma (HCC) due to their specific imaging characteristics and hypervascular behavior (17).
Additionally, we measured surrounding liver tissue attenuation coefficient values and registered eventual dilation of the biliary ducts, to find if there were changes in the density of perilesional parenchyma after SBRT treatment and if there was a relationship between SBRT treatment and the appearance of biliary dilation.
Methods
Lesions and patients
We selected 88 liver metastasis treated with SBRT at our institution, University Hospital HM Sanchinarro, Clara Campal Integral Oncology Center (CIOCC), Madrid (Spain), all of them with local control after at least one year of SBRT treatment (clinically and by imaging criteria). These lesions belonged to 39 patients with primary tumors of various origins (Table 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by our institutional ethics committee (No. 19.11.1463-GHM) at University Hospital HM Sanchinarro. Additional informed consent is not required according to our institutional ethics committee since all patients are anonymized and this is a data review study.
Table 1
| Characteristics | Number |
|---|---|
| Total number of lesions evaluated | 88 |
| Total number of patients | 39 |
| Age (years) | Median 64 (range, 41–81) |
| Sex | 56% male; 44% female |
| Primary tumors | 66% colorectal; 18% pancreatic; 8% breast; 8% other |
| Liver metastasis location | 81% peripheral; 19% central |
| Size of liver metastatic lesions before SBRT treatment (mm) | Mean 25 (range, 7–74) |
| Attenuation coefficient of liver metastatic lesions before SBRT treatment (HU) | Mean 56 (range, 18–101) |
| Attenuation coefficient of perilesional liver parenchyma before SBRT treatment (HU) | Mean 100 (range, 42–146) |
SBRT, stereotactic body radiation therapy.
SBRT treatment
All selected patients for our retrospective, observational study fulfilled the following characteristics: oligometastatic patient (five or less lesions), potentially treatable or controlled primary tumor, feasible placement of an internal marker, possibility of radical treatment with SBRT and adequate liver function with normal coagulation study.
For the accuracy of the treatment, we used as internal marker a gold thread of 0.75 mm ×30 mm (Visicoil®, IBA Dosimetry) which allowed an intrafraction control of liver motion. Placement of the internal markers was performed under CT control at the Radiology Department of our hospital, University Hospital HM Sanchinarro.
Once the internal marker was placed, simulation CT or PET-CT was performed (whether with the CT Siemens SOMATOM Sensation Open at the Radiation Oncology Department or with the Siemens Biograph 6 PET-CT Scanner), using a slice thickness of 3 mm and intravenous iodinated contrast. If simulation was performed with PET-CT, 18-fluoro-fluorodeoxyglucose (18F-FDG) was also administered at a dose of 370 MBq.
Images were sent in DICOM format to the TPS (Treatment Planification System), where hepatic metastasis and adjacent risk organs were outlined by the radiation oncologists to calculate the planning target volume (PTV), adding a margin of 5 mm to the gross tumor volume (GTV).
The radiophysicists performed the clinical dosimetries of hepatic SBRT treatments with the TPS iPlanRT Image® planning system or its previous versions iPlan RT v.3 and v.4 (Brainlab®). The type of dosimetry, 3-D conformal radiotherapy or intensity-modulated radiotherapy (IMRT), was determined based on the size and location of the lesion, as well as the proximity of organs at risk (OAR). Coplanar fixed fields were used, usually between 7 and 12 fields.
Doses of SBRT frequently prescribed were of 45–60 Gy in 3 fractions of 15–20 Gy, using doses of 50 Gy in 5 fractions of 10 Gy for metastasis very close to critical organs. The calculation algorithm used (PencilBeam) reliably reproduced the conditions of treatment of liver metastasis so that 95% of the prescribed dose covered 95% of the volume to be treated (PTV) and areas of overdosage, always if possible within the PTV, did not exceed 110%.
SBRT treatment of all lesions was performed in a Novalis® linear accelerator (Brainlab & Varian) at the Radiotherapy Oncology Department, a mono-energetic linear accelerator of 6 MV (MegaVolts) dedicated to Radiosurgery and SBRT treatments, with image guide radiation therapy (IGRT) system ExacTrac Adaptive Gating® (Brainlab) based on a robotic 6D coach, two X-ray tubes and two amorphous silicon screens that perform stereoscopic radiographs, capable of identifying bone structures or internal markers, which allowed intrafraction control of liver position and irradiation of the metastasis in a selected phase of the respiratory cycle.
Image analysis
We reviewed images of all CT and PET-CT studies performed before and every three months after SBRT treatment for the first year for each of the 88 lesions included: a total of 373 imaging studies corresponding to 246 CT and 127 PET-CT scans were analyzed. Lesions were delineated and measured by a radiologist with 15 years’ experience, gathering the size (cm) and “density” or attenuation coefficient values [UH, measured with a ROI (region of interest) in the centre of the lesion] of each metastasis and, if PET-CT was made, we also collected data concerning 18F-FDG uptake (SUVmax provided by our colleagues from Nuclear Medicine Department at our Hospital) to assess response according to different criteria (RECIST 1.1, Choi and PERCIST) (Table 2).
Table 2
| Response | RECIST 1.1 | Choi | PERCIST 1.0 |
|---|---|---|---|
| CR | Disappearance of lesions | Disappearance of lesions | Disappearance of 18F-FDG avid lesions |
| PR | ≥30% size decrease | ≥10% size decrease or ≥15% attenuation coefficient decrease | ≥30% 18F-FDG uptake decrease |
| SD | Neither PR nor PD | Neither PR nor PD criteria | Neither PR nor CR nor PD criteria |
| PD | ≥20% size increase or appearance of new lesions | ≥10% size increase without significant decrease of attenuation/appearance of new lesions or intratumoral nodules | ≥30% 18F-FDG uptake increase |
CR, complete response; PR, partial response; SD, stable disease; PD, progression disease.
Additionally, we measured perilesional liver tissue attenuation coefficient values (UH)—always in the same area in each revision—and registered eventual dilation of the biliary ducts (absent, mild, moderate, important or prior to SBRT treatment) to find if there were changes in the density of perilesional parenchyma after SBRT and if there was a relationship between treatment and appearance of biliary dilation, respectively.
All imaging studies after SBRT treatment were performed at our institution at portal phase, most of them at Toshiba Aquilion 64 CT Scanner at Radiology Department with a slice thickness of 1 mm, rotation time of 0.5 s, pitch of 0.8, modulated milliamperage and kilovoltage of 120 kilovolts (Kv), with 100 mL of intravenous iodinated contrast media with a flow rate of 3 mL/s. Many follow-up studies were obtained at the Siemens Biograph 6 PET-CT Scanner of Nuclear Medicine Department, and some at the CT Siemens SOMATOM Sensation Open at Radiation Oncology Department.
Each lesion was considered individually, regardless the behavior of other metastasis in the same patient or the eventual appearance of new lesions.
Statistical analysis
We used Statistical Package for the Social Sciences (SPSS) 21.0 (SPSS Inc., Chicago, EEUU) to collect and analyze all data.
The variation between the different criteria in the proportion of lesions assigned to each category of response: complete response (CR), partial response (PR), stable disease (SD) or progression disease (PD) was analysed by contingency analysis, using Yates correction when the sample size (number of lesions) at a time point (first, second, third or fourth trimester) was small. This test was also used to compare the sensitivity of PERCIST, RECIST and Choi criteria.
To analyze the effect of the location of the lesion on the caliber of the bile duct, since the biliary dilation variable was non-parametric, an analysis of generalized linear models (MLGz) (18) was used to study whether the location of the lesion affected the dilation of the bile duct. As the timing of the measurement was not a factor influencing the dilation of the biliary tract, dilation values of the four reviews were combined to perform a contingency analysis. To analyze if SBRT favored the appearance of dilation of the bile duct, we compared the frequency of patients who had some type of dilation of the bile ducts (mild, moderate, important) with the frequency of patients with pre-existing dilation, assuming that patients who had pre-existing dilation represented non-SBRT treatment causes. Finally, since the perilesional attenuation coefficient was a parametric variable, an analysis of general linear models (GLM) (18) was used to study if the attenuation coefficient varied depending on the “time point” of the measurement at consecutive reviews.
Results
Statistical analysis shown significant differences in the assessment of response of liver metastasis treated with SBRT using different criteria. In the four reviews (performed every three months after SBRT for the first year), the different criteria classified the response to treatment differently (P<0.001) (Figure 1).
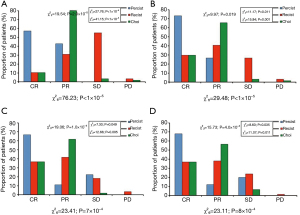
Data corresponding to revision 1 (performed the first three months after SBRT) of all lesions included were used to assess which criteria detected response earliest. The combined comparison of the three criteria in the first review indicated that the percentage of lesions classified as CR differed between criteria as well as with the observed value (P<0.001). The three criteria classified into CR category a percentage of patients less than the one observed (all lesions were controlled at least one year after SBRT) (P<0.001), although PERCIST classified correctly a greater percentage of lesions than the other two criteria throughout the first quarter (P<0.001), and also in the following revisions. PERCIST criteria were, therefore, the most sensitive to assess response in our study, detecting also this response earlier than the others. Regarding “non-metabolic” (“anatomical” or “morphologic”) criteria, Choi criteria showed better results in the assessment of response than RECIST 1.1 (Figures 1,2).
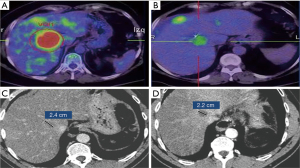
To analyze the effect of the location of the lesion on the caliber of the bile duct, since the biliary dilation variable was non-parametric, an analysis of MLGz (18) was used to study whether the location of the lesion affected the dilation of the bile duct. This analysis indicated that dilation of the bile duct varied according to the location of the lesion (P<0.001), being more than five times greater when the lesion was central (mean: 0.67±0.21) than when it was peripheral (mean: 0.12±0.04). On the other hand, no variation was detected in the dilation of the bile duct over the months (P=0.461), so as the timing of the measurement was not a factor influencing the dilation of the biliary tract, dilation values of the four reviews were combined to perform a contingency analysis comparing the frequency of the five categories of dilation (no dilation, mild dilation, moderate dilation, important dilation and pre-existing dilation) between central and peripheral lesions. This contingency analysis indicated that the absence of biliary dilation was more frequent in peripheral lesions than in central ones (P<0.001). To analyze if SBRT treatment favored the appearance of dilation of the bile duct, we compared the frequency of patients who had some type of dilation of the bile ducts (mild, moderate, important) with the frequency of patients with pre-existing dilation, assuming that patients who had pre-existing dilation represented non-treatment causes, whereas patients who developed dilation during the monitored period had causes that may be associated with SBRT treatment. Contingency analysis indicated that there was no difference between the frequency of patients who had pre-existing dilation and the frequency of patients who developed it after SBRT treatment, whether the lesion was central (P=0.330) or peripheral (P=0.522), so not significant differences were observed between SBRT and other treatments/conditions concerning bile ducts dilation (Figure 3).

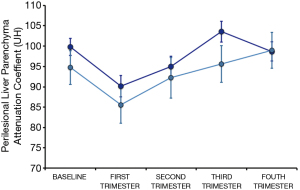
Finally, since the perilesional attenuation coefficient was a parametric variable, an analysis of GLM was used to study if the attenuation coefficient varied depending on the “time point” of the measurement at consecutive reviews. GLM analysis indicated that there were significant differences in the coefficient of attenuation according to the revision (P=0.036), so we analyzed these differences between pairs of revisions. For this we used the Least Significant Difference (LSD) test (18). Transitory decrease in liver attenuation coefficient values surrounding lesions was observed in the first three months after SBRT, when attenuation coefficient values were lower than in any of the other revisions, including baseline measurement (P<0.016), with no other significant changes during the first year of follow-up (Figure 4).
Discussion
SBRT is a safe and effective technique that has been developed very rapidly in recent years due to its excellent results in terms of tolerance and local control of injuries (4,19). Therefore, it is increasingly proposed as an alternative to radiofrequency and microwave ablation for local treatment of liver metastasis (8).
In our analysis, we considered each lesion treated with hepatic SBRT individually, as previously done by other authors such as Caivano et al. (20), and we demonstrated that there was significant variability in the classification of response to treatment based on the Criteria used. Thus, we observed significant differences in the way of classifying response to treatment between the three criteria used (PERCIST, RECIST and Choi) in the four reviews performed quarterly during the first year. All lesions included were already “in response” according PERCIST criteria in the first review (about 60% in CR and approximately 40% in PR) and only about 10% were in CR by RECIST 1.1 or Choi criteria (21). However, approximately 80% were in PR by Choi and only 30% by RECIST 1.1 in that first review. Therefore, Choi detected response earlier than RECIST, probably because it is necessary a smaller reduction in size than with RECIST to consider PR and because it takes into account changes in the attenuation coefficient values of the lesions, in addition to the size.
There are other therapies, besides those to treat GIST tumors for which Choi Criteria were developed, in which measurement of lesion attenuation coefficients has also been suggested to assess response to treatment. Thus, in antiangiogenic treatments it has already been shown that RECIST criteria, designed to assess response to cytotoxic treatments, are not adequate, as Chun observed that CRC metastasis, initially heterogeneous, with variable enhancement and poorly defined contour, after treatment with Bevacizumab transformed into homogeneous lesions, with low attenuation values and well-defined margins (11), so density changed after treatment.
Jarraya et al. (14) already suggested the use of “Combined Response Criteria”, that included changes in the attenuation and enhancement of hepatic lesions after SBRT treatment, in addition to size.
In all our analysis, PET-CT was the technique that detected response earlier, so we concluded that PERCIST Criteria were more adequate to assess response of liver metastasis treated by SBRT than RECIST 1.1 or Choi (Figures 1,2).
These results correspond to recent data published in the bibliography. Haddad et al. (7) reviewed the three behavior patterns by image of liver metastasis that respond to SBRT: decrease in 18F-FDG uptake, diminution of contrast enhancement and gradual reduction, throughout months, of size. This would explain the variability in the classification of response by different criteria along time, depending on whether a strictly anatomical or functional-metabolic assessment is made, and within the morphological or “non-metabolic” evaluation, whether if attenuation coefficient values are taking into account or not. Therefore it is logical that within morphological criteria Choi asses better response to SBRT than RECIST 1.1. Tétreau et al. have also recently published very similar findings and recommendations on evaluation liver tumors after SBRT (22).
Choi et al. (23) already used PET as a reference technique with which compare their new criteria. There are also several authors who have published that it is preferable to assess response by PET-CT when available than by CT, and that, when using CT, RECIST 1.1 criteria, based only on size, are insufficient (24). Until relatively recently, there was no clear consensus on how to perform PET-CT scans in different centers or how to assess PET-CT response, questions that have been lately resolved (25). Regarding radiation dose received by patients who underwent PET-CT studies, it is logically superior to that received in CT or PET studies performed individually. In any case, depending on acquisition technique, the contribution of CT to total dose in PET-CT studies ranges between 50% and 80% of the total (26), so when compared with CT studies differences between doses are generally small and, in some cases, non-existent. Availability of PET-CT equipments has increased lately, facilitating access to these examinations, although they are more expensive, but perhaps should be especially recommended in patients with contradictory findings on CT scans. MRI has already a relevant role to assess treatment response (7), fundamentally based on diffusion-weighted and contrast-enhanced sequences, but specific MRI criteria are not available yet.
Concerning dilation of the bile duct, in our series central lesions were associated with greater dilation of the bile duct than peripheral ones, although in no case this dilation was important. The percentage of patients in whom dilation of the biliary tract appeared throughout the follow-up was very similar to those who already had a dilatation of the bile duct before SBRT treatment, and therefore not attributable to it (Figure 3). So, in the absence of significant differences, it does not seem that SBRT causes dilation of the bile duct with greater probability than other treatments or causes; for example, Jhaveri et al. (27) demonstrated that colorectal carcinoma metastasis—the most frequent in our series—are associated with greater dilatation of the bile duct than other metastasis, due to their characteristic growth within the bile duct.
Olsen et al. (28) analyzed in their work pathological changes in the hepatic parenchyma after SBRT treatment, showing that hepatic radicals appeared to be respected, which would support our results, while long-term studies may be necessary to rule out delayed effects on the bile duct, although in our series there were no significant changes in the caliber of the bile duct over months.
Regarding changes in the attenuation coefficient values of hepatic parenchyma close to treated metastasis, already described by several authors (29,30), we observed that this attenuation coefficient values did not vary significantly along the first year except in the first revision, performed in the first three months after SBRT, when it was lower than in the rest—including baseline study. These transient changes could be partly due to “post-treatment perilesional edema” and other non-analyzed factors such as chemotherapy, which could also reduce the attenuation of liver parenchyma (Figure 4).
Conclusions
PERCIST were the most sensitive criteria to assess response to SBRT of liver metastasis in our series. Among non-metabolic criteria, Choi criteria were more appropriate than RECIST 1.1.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://amj.amegroups.com/article/view/10.21037/amj-21-17/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-21-17/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by our institutional ethics committee (No. 19.11.1463-GHM) at University Hospital HM Sanchinarro. Additional informed consent is not required according to our institutional ethics committee since all patients are anonymized and this is a data review study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326-32. [Crossref] [PubMed]
- Petrelli F, Comito T, Barni S, et al. Stereotactic body radiotherapy for colorectal cancer liver metastases: A systematic review. Radiother Oncol 2018;129:427-34. [Crossref] [PubMed]
- Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28-37. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol 2020;38:2830-8. [Crossref] [PubMed]
- Rubio Rodriguez C, Hernando-Requejo O, Zucca Aparicio D, et al. Image guided SBRT for multiple liver metastases with ExacTrac® Adaptive Gating | Cancerology Oncology. Reports of Practical Oncology and Radiother. Reports Pract Oncol Radiother. 2016;2016.
- NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed January 11, 2017.
- Haddad MM, Merrell KW, Hallemeier CL, et al. Stereotactic body radiation therapy of liver tumors: post-treatment appearances and evaluation of treatment response: a pictorial review. Abdom Radiol (NY) 2016;41:2061-77. [Crossref] [PubMed]
- Lee J, Shin IS, Yoon WS, et al. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: Meta-analyses and a systematic review. Radiother Oncol 2020;145:63-70. [Crossref] [PubMed]
- Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol 2007;25:1760-4. [Crossref] [PubMed]
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86. [Crossref] [PubMed]
- Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 2009;302:2338-44. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Jarraya H, Mirabel X, Taieb S, et al. Image-based response assessment of liver metastases following stereotactic body radiotherapy with respiratory tracking. Radiat Oncol 2013;8:24. [Crossref] [PubMed]
- Solanki AA, Weichselbaum RR, Appelbaum D, et al. The utility of FDG-PET for assessing outcomes in oligometastatic cancer patients treated with stereotactic body radiotherapy: a cohort study. Radiat Oncol 2012;7:216. [Crossref] [PubMed]
- Stinauer MA, Diot Q, Westerly DC, et al. Fluorodeoxyglucose positron emission tomography response and normal tissue regeneration after stereotactic body radiotherapy to liver metastases. Int J Radiat Oncol Biol Phys 2012;83:e613-8. [Crossref] [PubMed]
- Mendiratta-Lala M, Gu E, Owen D, et al. Imaging Findings Within the First 12 Months of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2018;102:1063-9. [Crossref] [PubMed]
- Robert R. Sokal and F. James Rohlf. Biometry: The Principles and Practice of Statistics in Biological Research; 1995.
- Rubio C, Morera R, Hernando O, et al. Extracranial stereotactic body radiotherapy. Review of main SBRT features and indications in primary tumors. Rep Pract Oncol Radiother 2013;18:387-96. [Crossref] [PubMed]
- Caivano D, Bracci S, Russo I, et al. Stereotactic Body Radiation Therapy for Liver Lesions. A Single-institution Experience. Anticancer Res 2015;35:4171-5. [PubMed]
- Choi H. Response evaluation of gastrointestinal stromal tumors. Oncologist 2008;13:4-7. [Crossref] [PubMed]
- Tétreau R, Llacer C, Riou O, et al. Evaluation of response after SBRT for liver tumors. Rep Pract Oncol Radiother 2017;22:170-5. [Crossref] [PubMed]
- Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753-9. [Crossref] [PubMed]
- Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med 2006;47:1059-66. [PubMed]
- Boellaard R, Oyen WJ, Hoekstra CJ, et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging 2008;35:2320-33. [Crossref] [PubMed]
- Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology 2009;251:166-74. [Crossref] [PubMed]
- Jhaveri KS, Halankar J, Aguirre D, et al. Intrahepatic bile duct dilatation due to liver metastases from colorectal carcinoma. AJR Am J Roentgenol 2009;193:752-6. [Crossref] [PubMed]
- Olsen CC, Welsh J, Kavanagh BD, et al. Microscopic and macroscopic tumor and parenchymal effects of liver stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:1414-24. [Crossref] [PubMed]
- Janoray G, Chapet S, Ruffier-Loubière A, et al. Robotic stereotactic body radiation therapy for tumors of the liver: radiation-induced liver disease, incidence and predictive factors. Cancer Radiother 2014;18:191-7. [Crossref] [PubMed]
- Herfarth KK, Hof H, Bahner ML, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys 2003;57:444-51. [Crossref] [PubMed]
Cite this article as: Allona Krauel M, Chen-Zhao X, Núñez Báez M, Hernando Requejo O, López de la Guardia U, Rubio Rodríguez C. Comparison between RECIST 1.1, Choi and PERCIST 1.0 criteria to evaluate response to SBRT of liver metastasis. AME Med J 2021;6:26.

