
Contemporary review: a clinically oriented interpretation of incidental radiological findings for common cardiovascular computed tomography scans
Introduction
Computed tomography (CT) angiography is widely used to assess coronary artery disease (CAD) and aortic conditions. CT angiography has undergone remarkable technological advancements in image quality, paralleled by an increasing recognition that CT angiography should be more broadly performed, serving as a non-invasive imaging tool for patients with cardiovascular disease. Incidental findings in the lung are common in radiology (1-3). Incidental benign lesions for example can lead to increased medical investigation costs (4), and cause potentially unnecessary psychological burden for the patient (5). Conversely, an incidental malignant lesion can expose serious underlying illnesses or generate additional diagnostic tests, which can lead to more therapeutic interventions that result in potential benefits pertaining to early treatment of serious asymptomatic conditions, such as cancer (4,6). Therefore, expert interpretation of incidental findings is important, because the patients and the treating physicians rely on accurate interpretation to decide on how these findings should best be managed.
This article addresses several key issues faced by cardiovascular imaging physicians who are primarily trained in the field of cardiology. For these physicians without dedicated training in radiology, it is even more important to be aware of these incidental findings that radiologists will usually be aware of due to their dedicated training.
Multidetector CT angiography techniques and protocols for cardiac imaging
Calcium score
Calcium score (CAC) has become a widely used preclinical screening tool to help clinicians make decisions about whether preventive medical therapies should be instigated, while providing an assessment of the likelihood of an individual patient receiving a net benefit from the preventive pharmacotherapies (7,8). The typical age of a patient with elevated atherosclerotic cardiovascular disease (ASCVD) risk without ischemic symptoms ranged between the ages 45 to 75 years. The preclinical screening tool creates an Agatston calcium score that allows the physician to identify potential cardiovascular risk and then provide essential information to patients. The Agatston score, a summed score base on the calcified plaque area and the maximal density of individual calcified lesions, has been the CAC metric of choice, and applied to ECG-gated study acquired with 120 kilovoltage (kV) at 2.5–3 millimeter (mm) slice thicknesses (9). Coronary calcium scan acquisition is achieved without physical intrusion because there is no intravenous contrast dose used in the procedure. The field of view (FOV) is focused on the heart and the scan range encompasses the carina through to the apex of the heart. Therefore, common incidental findings for this scan acquisition are found in the imaged portion of thorax (Table 1).
Table 1
| Type of scan | Details of the protocol | Incidental findings |
|---|---|---|
| Calcium score | ECG-gated acquisition. Scan range: carina to base of heart. Estimated radiation dose (10): 1–3 mSv |
Limited chest: pulmonary nodules; perifissural nodule; cystic lesions; fibrosis/scar; emphysema; mediastinal lymph node; hiatal hernia Breasts: calcifications Bone: vertebral hemangioma; enostosis (bone island); degenerative change |
| CT coronary angiography | Prospective/retrospective ECG-gated acquisition. Scan range: carina to base of heart; thoracic aperture to base of heart if patients underwent bypass graft. Estimated radiation dose (10): prospective ECG-gated technique: 2–4 mSv†. Retrospective ECG-gated technique: 8–18 mSv |
Limited neck: thyroid nodule. Limited chest: pulmonary nodules; perifissural nodule; cystic lesions; fibrosis/scar; emphysema; mediastinal lymph node; hiatal hernia Breasts: calcifications Bone: vertebral hemangioma; enostosis (bone island); degenerative change |
| Transcatheter aortic valve replacement (TAVR) protocol | Retrospective ECG-gated acquisition (dynamic 4D imaging). Scan range: upper thoracic aperture to the lesser trochanter. Estimated radiation dose (11): 16–24 mSv |
Limited neck: thyroid nodule. Limited chest: pulmonary nodules; perifissural nodule; cystic lesions; fibrosis/scar; emphysema; mediastinal lymph node; hiatal hernia Breasts: calcifications Bone: vertebral hemangioma; enostosis (bone island); degenerative change Abdomen‡: hepatic lesions; pancreatic lesions; renal stone; renal masses or cysts; gallstones; adrenal masses; adnexal masses |
†, in select patients with a small body surface area and stable low heart rates, use of high-pitch dual source helical scanning and low tube voltage have been reported to be associated with extremely low radiation dose [e.g., less than 1 mSv (12)]. ‡, incidental lesions in the abdomen are not the focus of the current review. CT, computed tomography; ECG, electrocardiogram; mSv, millisievert.
Coronary CT angiography
The 2019 European Society of Cardiology (ESC) guidelines for the diagnosis and management of chronic coronary syndromes recommend using coronary CT angiography as the main imaging modality to rule out CAD in patients who have non-diagnostic symptoms of obstructive CAD (class I recommendation, level B) (10). Conversely, the 2012 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for the diagnosis and management of stable ischemic heart disease recommend that a coronary CT be performed for patients who are unable to exercise. However, individuals who have equivocal exercise stress testing should have additional investigations. A prospective ECG-gated helical coronary CT with a multi-slice CT scanner is the standard technique (≥64 slices) (11). Various CT scanners and techniques have been created to improve the diagnostic accuracy for CAD findings, reduce contrast dose, and reduce radiation dose during the scan. The ECG-gated retrospective technique should be considered in patents with a high heart rate or an irregular heart rate; moreover, this method can be used to evaluate the cardiac and valve function (11). The FOV and scan range of this study are focused on the heart, an objective similar to the Agatston calcium score study. If the coronary CT angiography is performed to evaluate a coronary artery bypass graft; however, the FOV will need to be extended to the origin of internal mammary arteries, which are often only utilized as bypass grafts. Incidental findings in the imaged neck, breast, and thorax are encountered in this type of study (Table 1).
Transcatheter aortic valve replacement (TAVR) protocol
Developments in transcatheter-based therapies have provided an alternative therapeutic strategy for the non-surgical management of patients with severe symptomatic aortic stenosis. A CT is now the gold standard tool for preprocedural evaluation for TAVR (12), and it is also useful for post-procedural assessment and evaluation of potential complications (13). It is useful for assessing the aortic annular size and aortic annular plane, the quantity and distribution of calcium in the aortic valve, aortic valve morphology, device landing zone, and peripheral access (usually transfemoral) (13). The CT acquisition strategies and scanning protocols vary, reflecting the differences in scanners, imaging systems, and local expertise (11). Our institutional protocol requires performance of retrospective ECG-gated CTAs with 10 phases of reconstruction 0–100% (dynamic 4-dimensional imaging) from the carina through the apex of the heart and subsequently CTA from upper thoracic aperture to the lesser trochanter to include the thoracic and abdominal aorta, the iliac arteries, and common femoral arteries (Table 1).
Incidental findings
We searched MEDLINE publications from 1971 through August 2021 using the following key words: incidental findings and cardiac CT angiography.
Thyroid nodules
The incidental thyroid nodule (ITN) is defined as a nodule incidentally discovered, and not suspected to have occurred clinically, or previously known (14). ITNs are seen in up to 25% of CT scan (15). The malignancy rate of ITNs detected on CT is up to 10% (15).
There are no reliable signs that can differentiate between a benign thyroid nodule versus a malignant thyroid nodule on CT images (16,17). The largest diameter on axial CT images of thyroid nodules becomes the primary imaging feature (Figure 1) (15). Shetty et al. (16) reported patients aged ≤35 years, who had ITNs detected on CT, had a higher risk of malignancy. The American College of Radiology (ACR) incidental thyroid findings committee (14) recommend further evaluation with an ultrasound for patients who have certain clinical factors and imaging findings as shown in Table 2.
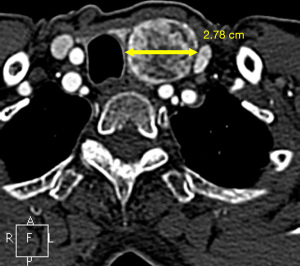
Table 2
| Thyroid nodules with suspicious features |
| Abnormal lymph node |
| Calcification |
| Cystic component |
| Increased enhancement |
| Size >1.5 cm in short axis for jugulodigastric lymph node or size >1 cm for other lymph nodes |
| Local invasion by thyroid nodule |
| Patients do not have serious comorbidity or limited life expectancy and |
| Age <35 years and thyroid nodule size ≥1 cm† |
| Age ≥35 years and thyroid nodule size ≥1.5 cm† |
| Heterogeneous, enlarged thyroid gland |
†, for multiple thyroid nodules, the criteria should be applied to the largest nodule.
Pulmonary nodules
Several studies have reported that an incidental pulmonary nodule was the most common incidental finding for cardiovascular CT examinations (18). Previous studies reported that the prevalence of significant pulmonary nodules such as lung cancer was up to 16% (19). Estimation of the probability of malignancy is essential for management. Both clinical parameters and features of pulmonary nodules are of importance. The Fleischner Society guidelines for pulmonary nodule management published in 2017 are widely adopted due to the precise recommendations based on patient risk, number of nodules, and nodule appearance (20). They also discuss the additional risk factors for malignancy; as a result, flexibility regarding follow-up imaging was added (20). The aforementioned guidelines are recommended for management of incidental pulmonary nodules found through CT imaging of patients aged >35 years with no history of cancers or immunodeficiency disorder. The management of incidental pulmonary nodules found in patients aged 35 years or younger should be individualized (20).
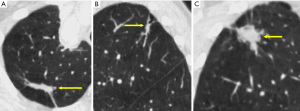
Evaluating the components of a pulmonary nodule is helpful for differentiating between benign and malignant lesions. Using both lung and soft-tissue window settings are recommended for nodule characterization (21). Attenuation measurement should be performed on the thinnest available non-sharp CT image (typically in the soft-tissue window), concentrating on the small region of interest and not only at a specific value point (20). The presence of fat [typically −40 to −120 Hounsfield units (HU)] within a lung nodule is sufficient to determine its benign nature (22). Visible calcification usually can be helpful for diagnosis. Presence of homogenous calcification, dense central calcification (bull’s eye calcification), concentric rings of calcium (target calcification), and conglomerate foci of calcification involving a large part of the nodule (popcorn calcification) generally indicates a benign lesion (22). The Fleischner Society guidelines categorize nodules into solid and subsolid nodules (20). Subsolid nodule is a term used for nodules that appear partially invisible when viewed on thin sections with mediastinal (soft-tissue) window setting and a sharp filter (20). Solid nodules appear as homogenous soft-tissue components other than normal vascular or bronchial structures on CT images (Figure 2A) (20,23). The subsolid nodules could be further divided into either ground-glass nodules or part-solid nodules. Ground-glass opacity represents hazy attenuation in the lung; however, the bronchial and vascular margins (Figure 2B) are preserved (23). A part-solid (synonym: semisolid) nodule consists of both ground-glass and solid soft-tissue attenuation components (Figure 2C) (23). Solid and ground-glass pulmonary nodules smaller than 6 mm generally do not need further evaluation or follow-up. Other pulmonary nodules should be followed-up with a dedicated interval CT, based on their appearances and sizes (Table 3) (20).
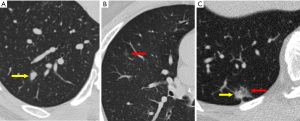
Table 3
| Type | Definition |
|---|---|
| Solid nodule (single) | |
| Size <6 mm | No suspicious morphology → no routine F/U |
| Suspicious morphologya → optional F/U CT chest at 12 months | |
| Size 6–8 mm | At incomplete CT → follow up with complete CT in 3–12 months or as early as possible if suspicious morphologya |
| Initial F/U at 6–12 months and reassessment of clinical riskb and if. Low risk and benign morphology at 12–18 months → discontinuing F/U; High risk, suspicious morphology, or uncertain stability → F/U at 18–24 months |
|
| Size >8 mm | At incomplete CT → further complete CT should be performed as early as possible |
| F/U CT at 3 months, work-up with PET/CT, tissue sampling, or combination | |
| Solid nodule (multiple)c | |
| Size <6 mm | Low risk → No routine F/U |
| High risk → F/U CT chest at 12 months may be considered | |
| Size ≥6 mm | Low risk → no routine F/U |
| High risk → F/U CT chest at 12 months may be considered | |
| Subsolid nodule (single) | |
| Ground-glass, size <6 mm | No suspicious morphology → No routine F/U |
| Close to 6 mm with suspicious morphologyd or other risk factor → optional F/U CT chest at 2 and 4 years | |
| Ground-glass, size ≥6 mm | Large lesion (especially >10 mm) and/or bubbly lucencies → initial F/U at 6 months |
| F/U at 6–12 months and then every 2 years thereafter until 5 years | |
| Part-solid, size <6 mm | No routine F/U |
| Part-solid, size ≥6 mm | F/U at 3–6 months: persistent lesion → yearly F/U for 5 years. Part-solid nodules with a solid component ≥6 mm → F/U at 3–6 months. Nodule with suspicious morphology (i.e., lobulated margin or cystic component), growing solid component, or a solid component >8 mm → PET/CT biopsy, or resection |
| Subsolid nodule (multiple) | |
| Size <6 mm | F/U at 3–6 months and if. Stable → F/U at 2 and 4 years. Progression → management base on dominant lesion |
| Size ≥6 mm | Management decision should be based on the most suspicious nodulee |
This table is modified and adapted from ref (20) with permission from Radiological Society of North America. a, suspicious morphology of solid nodule included located in upper lobe, contour irregularity or spiculation. b, high clinical risk included, old age and heavy smoking (≥30 pack-years and quitting within 15 years). c, use clinical risk and the most suspicious nodule (which may not be the largest) as guide to management. d, suspicious morphology of ground-glass nodule included spiculation and fissure distortion. e, interval growth of a solid component that is ≥6 mm, spiculated margins, interval increase in density, and a new microcystic component are some of the most important features used to defined the most suspicious lesion. mm, millimeter; F/U, follow-up; CT, computed tomography; PET, positron emission tomography.
For measurement of the nodule size, the diagnostic image should be displayed using the lung window setting with a high-spatial-frequency (sharp) algorithm and contiguous thin sections (≤1.5 mm, typically 1.0 mm) (24,25). The measurement should be performed on the transverse section, unless the maximal dimensions lie in a coronal or sagittal plane, which should be documented in the report (21). Volumetric measurement is also used to assess nodule growth, but can be limited by variations in CT interpretation software package versions (25,26). Nodules smaller than 3 mm should generally not be measured due to accuracy limitation, and can be described as “micronodules” (23). All measurements should be expressed to the nearest whole millimeter. The size of the solid nodule and ground-glass nodule should be recorded as the average of the maximal long-axis diameter and the maximal short-axis diameter, perpendicular to the long-axis measurement for nodules smaller than 10 mm. For larger nodules, recording both the long-axis dimension and the short-axis dimension is recommended.
The recommended approach for spiculated nodules is to measure only the solid core (Figure 3). The part-solid nodule measurement requires evaluation of both the overall presence of lesions and the solid component in the nodules (Figure 4). The overall size is approached in the same manner as solid nodules. The solid component should be measured in the maximal diameter and should be reported if larger than 3 mm (22,24).
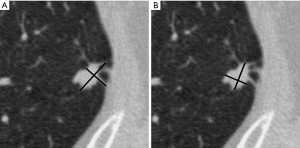
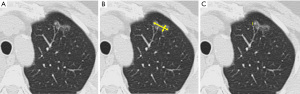
The measurements at follow-up CT should be acquired with similar techniques and orientations. They can be made through the centroid of the nodules, which may not be at the same anatomic level. Nodule growth should be determined by an increase in size of 2 mm or more for the overall size or the solid component of a part-solid nodule (21). Potential growth must be assessed in the context of the time interval between serial CT examinations. The accuracy of growth assessment improves with increasing intervals between examinations (27); and therefore, all prior studies should be used for comparison. Recommendations for management of incidental pulmonary nodules from Fleischner Society (21,22,28) are summarized in Table 3. The initial short-term follow-up recommended for part-solid nodules 6 mm or larger is important. The resolution of part-solid nodules is reassuring to patients and physicians. Stable pure ground-glass nodules likely represent adenomatous hyperplasia (29). Persistent part-solid nodules with a solid component smaller than 6 mm typically are adenocarcinomas in situ or minimally invasive adenocarcinomas (28). The solid component of a part-solid lesion is indicative of an invasive component. A decrease in the size of the nodule on follow-up, accompanied by an increased density, does not always indicate a benign lesion; however, careful recognition of the appearance of the nodule is important to avoid misinterpretation, diagnosis, and unwarranted treatment (28).
Perifissural nodules
Even though data on perifissural nodules on cardiac CT are limited, perifissural nodules are common findings on chest CT (30). Perifissural nodules are used to describe small solid nodules which are commonly seen on CT images adjacent to pleural fissures, representing intrapulmonary lymph nodes (20). There is generally no routine need for a follow-up CT for perifissural nodules, which have a triangular or lentiform morphology with a smooth contour, sharp margins, and a fine linear septal extension to the pleura (20). If perifissural nodules have certain features, including a round, irregular, or spiculated morphology; or an abnormal fissure (i.e., retracted, displaced, bowed, or transgressed fissure), follow-up CT at 6–12 months is suggested (20,28).
Cystic lesions
Cystic lesions with a thin (usually <2 mm) smooth wall are benign cysts (28). Cystic lesion associated with primary lung cancers demonstrate suspicious features including a mural nodule, or new microcysts in a solid or subsolid nodule, and progressive or asymmetric wall thickening (28).
Fibrosis/scar
Fibrosis and scar sometimes mimic spiculated nodules. Pleural-based configuration, and elongated shape, straight, or concave margins, and presence of similar adjacent opacities are suggestive of a scar (20). Assessment with multiplanar reconstructions is helpful (Figure 5), but in most cases, the scar is benign. Tuberculosis is the most common cause of lung lobe scars in developing country (31); whereas, idiopathic pulmonary fibrosis is the most common cause in developed country (32). Lung scar carcinoma is very rare, as first described by Friedrich in 1939 (33). A recent study showed that scar-tissue changes, such as enlargement and surrounding ground-glass opacification, can possibly be early signs of lung cancer that has developed from an existing scar (34). Thus, all regions of scars/fibrosis should be compared with prior CT studies, if available.
Emphysema
Emphysema is characterized by permanently enlarged air-spaces distal to the terminal bronchioles with destruction of alveolar walls (35). Emphysema is usually classified into three subtypes, based on the anatomic distribution or the areas of lung destruction: (I) centrilobular and proximal acinar; (II) paraseptal and distal acinar; and (III) panlobular or panacinar and whole acinar (23,24). The appearances on CT imaging as a result of emphysema include focal or regional areas of low attenuation, usually without a definite visible wall (36). A prior study found the presence of coronary lesions on coronary CT significantly correlated with the presence of pulmonary emphysema (37) and emphysema-like lung conditions (38).
Mediastinal lymph nodes
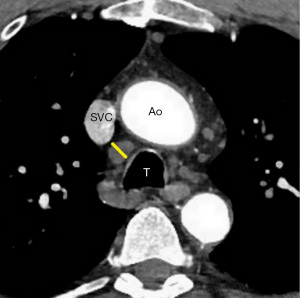
Normal mediastinal lymph nodes are generally considered to be up to 10 mm in short axis diameter (Figure 6) (39). Prior studies (40,41) demonstrated mediastinal lymphadenopathy ≤15 mm usually resulted from a benign etiology. The features of benign lymph nodes include well-demarcated, smooth borders, uniform and homogeneous attenuation, and a central fatty core (42). The absence of these features raises suspicion for a clinically concerning condition such as lymphoma. The current American College of Radiology (ACR) Incidental Findings Committee recommendations for managing incidentally detected mediastinal lymph nodes on CT (43) suggest that generally no further work-up for lymph nodes with short axis diameter ≤15 mm with benign features (43) is required. For lymph nodes larger than 15 mm, management is dependent on etiology, and in any case of uncertainty, it should be correlated with the patient’s clinical history, to assess the need for potential further evaluation (43).
Breast lesions
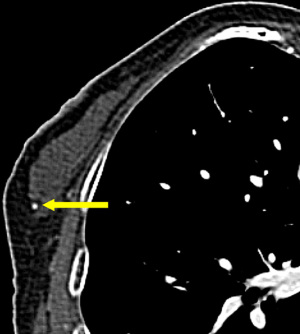
Given the limited spatial resolution of current CT scanners (0.5–0.625 mm on the z-axis; approximately 0.5 mm on the x- and y-axes) almost all breast calcifications detected on CT are benign (Figure 7) (44); in comparison, there is a high probability that malignant calcifications will be smaller than 0.5 mm) (Figure 8). In recent years, many studies have attempted to demonstrate the significant malignant features of incidental breast lesions on CT, usually combining lesion morphology and pattern of enhancement based on dynamic CT technique (45,46). Cardiac protocols mainly focus on the arterial phase and is generally not enough for evaluating enhancement of the breast lesion (47). CT scans may occasionally allow for a precise diagnosis of a previously unsuspected breast lesion, but mammography is usually required for definitive diagnosis (46).
Hiatal hernia
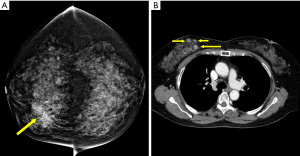
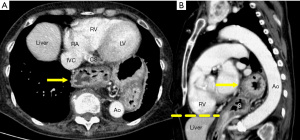
Most hiatal hernias are found incidentally. A hiatal hernia frequently occurs in individuals aged 50 years and above, and any individual who is overweight. These clinical features are also the same risk factors for CAD (48,49). Some people with hiatal hernias may experience chest pain or chest discomfort, which may mimic ischemic heart disease (50). A hiatal hernia appears as a retrocardiac mass, with or without air-fluid levels, which can be traced from the esophagus to the stomach (Figure 9). The management of the hiatal hernia depends on the patient’s symptoms, ranging from observation to medical therapies to potentially surgery in patients with severe symptoms and large hernias.
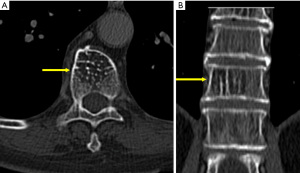
Vertebral hemangioma
Vertebral hemagiomas are the most common primary tumors of the spine; and usually, incidental findings are often found on CT and MR imaging (51,52). Most vertebral hemangiomas are small and asymptomatic (51). A coarse, vertical trabecular pattern (corduroy or polka-dot appearance) is identified in the vertebral body and may extend into the pedicles and laminae (Figure 10) (51). Extension of the lesion into the paraspinal soft tissue and spinal canal may be evident, and result in radiographic abnormalities that mimic a malignant neoplasm (51).
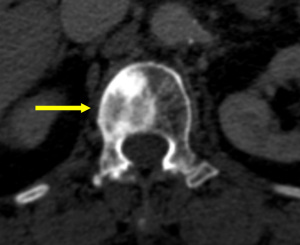
Enostosis
Enostosis, also known as bone island, is commonly found incidentally on imaging studies. However, this benign lesion may mimic osteoblastic metastasis (52). Enostosis is considered to be homogenous with a density similar to cortical bone on CT, for it is composed of compact bone while sclerotic metastasis may show less-homogenous internal attenuation than cortical bone (Figure 11) (53). It is challenging to differentiate between a small single enostosis from a sclerotic metastasis using CT. without multifocal identifiable lesions. On serial imaging, as many as 31% of enostosis will change in size (54). If the diameter of the growth is less than 25% in size over 6 months, a biopsy should not be required; however, if there is an increase of 50% or greater in 1 year (53), a biopsy should be considered (55).
Degenerative changes of spine
Degenerative changes of the spine affect the physical well-being of most individuals in the later stages of their life. There are several conditions that have a direct impact on the quality of life of older patients, such as disc height reduction, osteophyte, endplate sclerosis, and especially for older patients. These common but debilitating conditions can be considered as normal stages of aging (56). If these findings were discovered in younger patients, they should be reported because they may highlight underlying clinical conditions (56).
Summary
We provide a contemporary review of CT techniques and protocols for cardiac imaging and how to interpret common incidental findings. Once an examination is performed, the optimal approach to evaluation of incidental findings is to view and evaluate all available data, and then apply sound clinical judgment, in the best interest of each patient. Discussions regarding the recommendations from guidelines and clinical practice in this article should be adapted according to local standards of care, expertise, and patient populations.
Acknowledgments
The authors would like to acknowledge Mr. Gregory Alan Smith of the Naresuan University Language Centre for his editing assistance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-21-30/coif). BX serves as an unpaid editorial board member of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mueller J, Jeudy J, Poston R, et al. Cardiac CT angiography after coronary bypass surgery: prevalence of incidental findings. AJR Am J Roentgenol 2007;189:414-9. [Crossref] [PubMed]
- Colletti PM. Incidental findings on cardiac imaging. AJR Am J Roentgenol 2008;191:882-4. [Crossref] [PubMed]
- Kawano Y, Tamura A, Goto Y, et al. Incidental detection of cancers and other non-cardiac abnormalities on coronary multislice computed tomography. Am J Cardiol 2007;99:1608-9. [Crossref] [PubMed]
- Lee CI, Tsai EB, Sigal BM, et al. Incidental extracardiac findings at coronary CT: clinical and economic impact. AJR Am J Roentgenol 2010;194:1531-8. [Crossref] [PubMed]
- Schlett CL, Rospleszcz S, Korbmacher D, et al. Incidental findings in whole-body MR imaging of a population-based cohort study: Frequency, management and psychosocial consequences. Eur J Radiol 2021;134:109451. [Crossref] [PubMed]
- Machaalany J, Yam Y, Ruddy TD, et al. Potential clinical and economic consequences of noncardiac incidental findings on cardiac computed tomography. J Am Coll Cardiol 2009;54:1533-41. [Crossref] [PubMed]
- Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: Coronary Artery Calcium Data and Reporting System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr 2018;12:185-91. [Crossref] [PubMed]
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e563-95. [Crossref] [PubMed]
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827-32. [Crossref] [PubMed]
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-77. [Crossref] [PubMed]
- Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016;10:435-49. [Crossref] [PubMed]
- Xu B, Gooley R, Seneviratne SK, et al. Clinical utility of multi-detector cardiac computed tomography in structural heart interventions. J Med Imaging Radiat Oncol 2016;60:299-305. [Crossref] [PubMed]
- Blanke P, Weir-McCall JR, Achenbach S, et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc Imaging 2019;12:1-24. [Crossref] [PubMed]
- Hoang JK, Langer JE, Middleton WD, et al. Managing incidental thyroid nodules detected on imaging: White paper of the ACR incidental thyroid findings committee. J Am Coll Radiol 2015;12:143-50. [Crossref] [PubMed]
- Nguyen XV, Choudhury KR, Eastwood JD, et al. Incidental thyroid nodules on CT: evaluation of 2 risk-categorization methods for work-up of nodules. AJNR Am J Neuroradiol 2013;34:1812-7. [Crossref] [PubMed]
- Shetty SK, Maher MM, Hahn PF, et al. Significance of incidental thyroid lesions detected on CT: correlation among CT, sonography, and pathology. AJR Am J Roentgenol 2006;187:1349-56. [Crossref] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Iribarren C, Hlatky MA, Chandra M, et al. Incidental pulmonary nodules on cardiac computed tomography: prognosis and use. Am J Med 2008;121:989-96. [Crossref] [PubMed]
- Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol 2013;201:555-64. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Bankier AA, MacMahon H, Goo JM, et al. Recommendations for Measuring Pulmonary Nodules at CT: A Statement from the Fleischner Society. Radiology 2017;285:584-600. [Crossref] [PubMed]
- Webb WR, Higgings CB. Thoracic Imaging: Pulmonary and Cardiovascular Radiology. 2nd ed. Mitchell CW, editor. Lipppincott Williums and Wilkins, 2011.
- Hall FM. Fleischner Society glossary of terms: infiltrates. Radiology 2008;248:1083. [PubMed]
- Lehman SJ, Abbara S, Cury RC, et al. Significance of cardiac computed tomography incidental findings in acute chest pain. Am J Med 2009;122:543-9. [Crossref] [PubMed]
- Ashraf H, de Hoop B, Shaker SB, et al. Lung nodule volumetry: segmentation algorithms within the same software package cannot be used interchangeably. Eur Radiol 2010;20:1878-85. [Crossref] [PubMed]
- de Hoop B, Gietema H, van Ginneken B, et al. A comparison of six software packages for evaluation of solid lung nodules using semi-automated volumetry: what is the minimum increase in size to detect growth in repeated CT examinations. Eur Radiol 2009;19:800-8. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-ii54. [Crossref] [PubMed]
- Bueno J, Landeras L, Chung JH. Updated Fleischner Society Guidelines for Managing Incidental Pulmonary Nodules: Common Questions and Challenging Scenarios. Radiographics 2018;38:1337-50. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent Pure Ground-Glass Opacity Lung Nodules ≥ 10 mm in Diameter at CT Scan: Histopathologic Comparisons and Prognostic Implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Mets OM, Chung K, Scholten ET, et al. Incidental perifissural nodules on routine chest computed tomography: lung cancer or not? Eur Radiol 2018;28:1095-101. [Crossref] [PubMed]
- Bobba RK, Holly JS, Loy T, et al. Scar carcinoma of the lung: A historical perspective. Clin Lung Cancer 2011;12:148-54. [Crossref] [PubMed]
- Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180076. [Crossref] [PubMed]
- Friedrich G. Periphere Lungenkrebse auf dem Boden pleuranaher Narben. Virchows Arch Pathol Anat Physiol Klin Med 1939;304:230-47. [Crossref]
- Gao F, Ge X, Li M, et al. CT features of lung scar cancer. J Thorac Dis 2015;7:273-80. [PubMed]
- Thurlbeck WM, Müller NL. Emphysema: definition, imaging, and quantification. AJR Am J Roentgenol 1994;163:1017-25. [Crossref] [PubMed]
- Foster WL Jr, Gimenez EI, Roubidoux MA, et al. The emphysemas: radiologic-pathologic correlations. Radiographics 1993;13:311-28. [Crossref] [PubMed]
- Kitamura A, Okafuji K, Nakaoka H, et al. Prevalence and clinical significance of pulmonary disease found incidentally by coronary CT. Eur Respir J 2016;48:PA3817.
- Oelsner EC, Hoffman EA, Folsom AR, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med 2014;161:863-73. [Crossref] [PubMed]
- Hansell DM, Lynch DA, McAdams HP, et al. Imaging of Diseases of the Chest. 5th ed. Mosby Elsevier, 2010:69 p.
- Stigt JA, Boers JE, Oostdijk AH, et al. Mediastinal incidentalomas. J Thorac Oncol 2011;6:1345-9. [Crossref] [PubMed]
- Evison M, Crosbie PA, Morris J, et al. A study of patients with isolated mediastinal and hilar lymphadenopathy undergoing EBUS-TBNA. BMJ Open Respir Res 2014;1:e000040. [Crossref] [PubMed]
- Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging. J Nucl Med 2004;45:1509-18. [PubMed]
- Munden RF, Carter BW, Chiles C, et al. Managing Incidental Findings on Thoracic CT: Mediastinal and Cardiovascular Findings. A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2018;15:1087-96. [Crossref] [PubMed]
- Boone JM, Nelson TR, Lindfors KK, et al. Dedicated breast CT: radiation dose and image quality evaluation. Radiology 2001;221:657-67. [Crossref] [PubMed]
- Miyake K, Hayakawa K, Nishino M, et al. Benign or malignant?: differentiating breast lesions with computed tomography attenuation values on dynamic computed tomography mammography. J Comput Assist Tomogr 2005;29:772-9. [Crossref] [PubMed]
- Goldberg PA, White CS, McAvoy MA, et al. CT appearance of the normal and abnormal breast with mammographic correlation. Clin Imaging 1994;18:262-72. [Crossref] [PubMed]
- Harish MG, Konda SD, MacMahon H, et al. Breast lesions incidentally detected with CT: what the general radiologist needs to know. Radiographics 2007;27:S37-51. [Crossref] [PubMed]
- Pencina MJ, Navar AM, Wojdyla D, et al. Quantifying Importance of Major Risk Factors for Coronary Heart Disease. Circulation 2019;139:1603-11. [Crossref] [PubMed]
- Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc 2013;27:4409-28. [Crossref] [PubMed]
- Schummer W. Hiatal hernia mimicking heart problems. BMJ Case Rep 2017;2017:bcr-2017-220508. [Crossref] [PubMed]
- Resnick D, Kransdorf MJ. Bone and joint imaging. 3rd ed. Pennsylvania: Elsevier Inc., 2005.
- Gould CF, Ly JQ, Lattin GE Jr, et al. Bone tumor mimics: avoiding misdiagnosis. Curr Probl Diagn Radiol 2007;36:124-41. [Crossref] [PubMed]
- Bernard S, Walker E, Raghavan M. An Approach to the Evaluation of Incidentally Identified Bone Lesions Encountered on Imaging Studies. AJR Am J Roentgenol 2017;208:960-70. [Crossref] [PubMed]
- Onitsuka H. Roentgenologic aspects of bone islands. Radiology 1977;123:607-12. [Crossref] [PubMed]
- Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol 1991;20:85-90. [Crossref] [PubMed]
- Weckbach S. editor. Incidental Radiological Findings. Heidelberg: Springer International Publishing, 2017.
Cite this article as: Sripariwuth A, Kruamak T, Xu B. Contemporary review: a clinically oriented interpretation of incidental radiological findings for common cardiovascular computed tomography scans. AME Med J 2022;7:9.

