
Efficacy and safety of neoadjuvant immunotherapy in non-small cell lung cancer: a systematic review and meta-analysis
Highlight box
Key findings
• Neoadjuvant immunotherapy combined with chemotherapy has preferable efficacy and acceptable safety. Patients with high PD-L1 expression [tumor proportional score (TPS) >50%] are more likely to benefit from neoadjuvant immunotherapy.
What is known and what is new?
• Neoadjuvant immunotherapy displays high pathologic response rate and admissible side effects.
• Neoadjuvant immunotherapy combined with chemotherapy shows better effectiveness and acceptable safety compared with immunotherapy alone. PD-1 inhibitors are preferable. PD-L1 expression level and radiologic response associate with pathologic response closely.
What is the implication, and what should change now?
• These data provide evidence for treatment decisions. Combined therapy and patients with high PD-L1 expression are preferred.
Introduction
Lung cancer ranks first in morbidity and mortality in males among all malignant tumors worldwide (1), of which non-small cell lung cancer (NSCLC) accounts for nearly 80%. Surgery is always the basic treatment of early-stage NSCLC (2). However, the 5-year survival rate ranges from 36% to 92% with a high probability of recurrence, especially distant metastasis (3). Recently, researchers applied chemotherapy before surgery and achieved elevation in progression-free survival (PFS) and overall survival (OS) (4). However, with the development of neoadjuvant therapy, surgery, and adjuvant therapy, the prognosis is still not satisfactory. As we know, only a 5% improvement in the 5-year survival rate is observed (5). Also, accompanied by improved efficacy, adverse events happened more frequently.
Recently, immune checkpoint inhibitors (ICIs), a kind of antitumor drug, have been proven to have satisfactory effects in advanced NSCLC (6,7). The high response rate in stage IV NSCLC urges researchers to explore the efficacy and safety of ICIs in early-stage NSCLC. In recent years, many trials have been conducted such as LCMC3, NEOSTAR, NEOMUN, and so on. Studies showed exciting short-term outcomes. However, side effects occurred frequently. In NADIM, 93% of the participants receiving chemotherapy and ICIs underwent side effects and 30% were 3–5 grade treatment-related adverse event (TRAE). ChiCTR-OIC-17013726 also reported one grade 5 pneumonitis related to sintilimab. Consequently, we needed to analyze the efficacy and safety of neoadjuvant immunotherapy and combination strategies. Regrettably, most trials with data published online are phase 2 and single-arm studies, reporting short-term results. Few long-term survival data have been published (8). A previous study showed that histopathologic response related strongly to long-term OS. As a result, we utilized pathologic response and radiologic response as surrogate end points (9,10). Our meta-analysis aims to integrate the clinical data and pathologic data of recently published studies, predict survival and give evidence to guide clinical practice. We present the following article in accordance with the PRISMA reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-88/rc).
Methods
Search strategy and study selection
PubMed, OVID, and Cochrane Library were searched to access comprehensive studies with keywords including “NSCLC”, “neoadjuvant immunotherapy” and “trials”. The deadline for the search strategy is October 1, 2021. Please refer to the Appendix 1 for a detailed search strategy. On the other hand, due to the small number of published trials related, we also searched international tumor conferences such as American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), World Conference on Lung Cancer (WCLC), and other recent congresses. Thus, studies with abstracts only were also in our search list.
The population, intervention, comparator, outcome, and study (PICOS) criteria were followed and the inclusion criteria were listed as follows: (I) patients: resectable stage I–III NSCLC; (II) intervention: neoadjuvant ICIs; (III) comparator: how effective and safe are the different combinations and types of neoadjuvant treatment regimens; (IV) outcomes: objective response rate (ORR), surgical resection rate, R0 surgical resection rate (R0 rate), the incidence of major pathological response (MPR), pathological complete response (pCR), TRAE, 3–5 grade TRAE, surgical complications, and surgical delay, etc.; (V) study design: randomized controlled trials (RCTs), non-RCTs, prospective cohort studies. Those treated with any ICIs or radiotherapy previously should be excluded. Studies not focusing on the efficacy or safety of neoadjuvant immunotherapy were also rejected. Furthermore, the search also refused reviews and case reports.
Two reviewers (XK and WXD) were assigned to screen the title and abstract of each study independently. Then, we investigated full texts of studies included after the first search round. For those with disagreements, the two reviewers discussed together or asked for the third reviewer to decide on the final inclusion.
Data abstracted
Two reviewers (XK and WXD) separately extracted detailed data. Data containing the first author, published year, name of the trial, registration number, intervention type, drug and dose, number of enrollment, and baseline characteristics of participants. Short-term outcomes such as ORR, MPR, pCR, surgical resection rate, R0 rate, TRAE, 3–5 grade TRAE, the incidence of surgical complications, and surgical delay rate were recorded. Furthermore, we extracted the detailed baseline data of patients with MPR or pCR in each trial. Nevertheless, few studies supplied survival outcomes, and some results called for calculation, so we recorded the comprehensive raw data as possible. The two reviewers read studies repeatedly to make sure the accuracy and authenticity of the recorded data.
Statistical analysis
R version 4.1.1 was applied to perform the statistical analysis. The R META package was used for the meta-analysis and meta-regression. For proportions, the R function METAPROP was applied. For the raw data, simple calculations were done and the most suitable method was chosen to ensure its normality when using the METAPROP function. All included studies were combined in a descriptive synthesis. The heterogeneity was assessed using I2 and T2 values and calculated the estimates for the aforementioned clinical outcomes together with their 95% confidence intervals (CIs). Determination of which model to use to calculate the estimated values was made on both the authors’ assessment and statistical heterogeneity. Funnel plots were created to evaluate the publication bias of each included study. Egger’s test and Begg’s test were used to check for publication bias. In consideration of the limited trials included in this study, meta-regression was applied to explore the possible relationship between the duration time of surgery and MPR. Furthermore, due to the large heterogeneity, subgroup analysis was conducted based on the questions raised below: whether ICIs and chemotherapy (immunotherapy and chemotherapy or immunotherapy) should be combined and which type of ICIs [programmed cell death protein 1 (PD-1) inhibitor or programmed cell death protein ligand 1 (PD-L1) inhibitor] was preferred.
Moreover, the R function of METABIN was applied to explore the relationship between gender, smoking status, clinical stage, radiologic response, PD-L1 expression level, and pathologic response. Relative risk (RR) and 95% CI were the effective measures. Heterogeneity was assessed using the I2 and t2 values. The random effect model was adopted when results had significant heterogeneity; otherwise, we chose the fixed effect model.
All reported P values were two-sided and statistical significance was defined as P<0.05.
Assessments of publication bias and study quality
Because almost all the studies are non-randomized clinical trials except CheckMate 816, the Methodologic Index for Nonrandomized Studies (MINORS) was applied to assess the bias of these trials. Two reviewers independently carried out the quality evaluation. When encountering disagreement, the resolution strategy was the same as that conducted before.
Results
Results of search
After an advanced search of 3 databases (PubMed, OVID, and Cochrane Library), 487 studies were retrieved from the first round. Together with 215 studies obtained from other sources, 702 studies were included in the total. Following the removal of 226 duplicate articles, we excluded 444 articles by browsing titles and abstracts. Thirty-two articles went into the last round. Then 14 articles were excluded after viewing the full text. They were excluded for different main points or updated data. The study selection process is shown in Figure 1. Ultimately, 18 articles (8,11-27), with 859 patients were included in the final meta-analysis. Table 1 provides detailed information on the 18 records (19 trials) involved. As we can see in the table, only one phase 3 dual-arm open-label RCT, CheckMate 816, is included in the final meta-analysis.
Table 1
| First author | Year | Clinical trail | NCT number | Phase | ICIs | Main inclusion criteria | Enrollment | Cycles | Median age (years) |
|---|---|---|---|---|---|---|---|---|---|
| Shugeng Gao | 2020 | ChiCTR-OIC-17013726 | – | Ib | Sintilimab | IA–IIIB | 40 | 2 | 62 |
| B. Besse | 2020 | PRINCEPS | NCT02994576 | 2 | Atezolizumab | IA–IIIA | 30 | 1 | 64 |
| David P. Carbone | 2020 | LCMC3 | NCT02927301 | 2 | Atezolizumab | IB–IIIB | 181 | 2 | 65.1 |
| Catherine A Shu | 2020 | MAC | NCT02716038 | 2 | Atezolizumab | IB–IIIA | 30 | 4 | – |
| Mariano Provencio | 2020 | NADIM | NCT03081689 | 2 | Nivolumab | IIIA | 46 | 3 | 63 |
| Tina Cascone | 2021 | NEOSTAR | NCT03158129 | 2 | Nivolumab | IA–IIIA | 23 | 3 | 65.6 |
| Tina Cascone | 2021 | NEOSTAR | NCT03158129 | 2 | Nivolumab + ipilimumab | IA–IIIA | 21 | 3 | 65.6 |
| Ralph Zinner | 2020 | – | NCT03366766 | 2 | Nivolumab | IB–IIIA | 13 | 1 | 69 |
| Jonathan Spicer | 2021 | CheckMate 816 | NCT02998528 | 3 | Nivolumab | IB–IIIA | 179 | 3 | – |
| P. M. Forde | 2018 | CheckMate 159 | NCT02259621 | 1 | Nivolumab | I–IIIA | 21 | 2 | 67 |
| Chi-Fu Jeffrey Yang | 2017 | TOP1201 IPI | NCT01820754 | 2 | Ipilimumab | II–IIIA | 24 | 2 | 65 |
| Zerui Zhao | 2021 | NeoTPD01 | NCT04304248 | 2 | Toripalimab | IIIA–IIIB | 33 | 3 | 61 |
| M. Wislez | 2020 | IONESCO | NCT03030131 | 2 | Durvalumab | IB–IIIA | 46 | 3 | 61 |
| Sacha I. Rothschild | 2021 | SAKK 16/14 | NCT02572843 | 2 | Durvalumab | IIIA | 67 | 2 | – |
| N. Ready | 2019 | MK3457-233 | – | NR | Pembrolizumab | IB–IIIB | 30 | 2 | – |
| A. Ben Nun | 2019 | MK3475−223 | NCT02938624 | 1 | Pembrolizumab | I–II | 10 | 2 | 70.5 |
| Florian Eichhorn | 2021 | NEOMUN | NCT03197467 | 2 | Pembrolizumab | II–IIIA | 15 | 2 | 59.8 |
| TOP1501 | 2021 | TOP1501 | NCT02818920 | 2 | Pembrolizumab | IB–IIIA | 35 | 2 | 71 |
| Y. Zhang | 2021 | – | MCT4144608 | NR | Toripalimab | IIIA–IIIB | 15 | 2 | 57 |
NSCLC, non-small cell lung cancer; ICIs, immune checkpoint inhibitors; NR, not recorded.
Primary outcomes
Efficacy of neoadjuvant immunotherapy
ORR is defined as a radiologic response according to the RECIST version 1.1 criteria (28,29). Fourteen studies reported specific data on ORR (Figure 2). The mean result was 39.1% (95% CI, 24.3–54.0%). In the pooled surgical resection rate, the percentage of patients who successfully underwent surgery was 90.7% (95% CI, 85.3–95.1%) of the 19 trials involved. As for the R0 rate, the 15 trials’ average result was 97.8% (95% CI, 94.8–99.6%). In terms of pathologic response, we applied MPR and pCR as the research objects. The definition of MPR was less than 10% of viable tumor cells in both resected primary tumor beds and lymph nodes. On this basis, pCR was completely absent of viable tumor cells. Pooled MPR based on 15 studies was 44.4% (95% CI, 29.9–59.4%) and pCR of 16 trials involved reached 23.3% (95% CI, 14.4–32.2%) (Figure S1).

Safety of neoadjuvant immunotherapy
We obtained specific information on the incidence of TRAE, the incidence of 3–5 grade TRAE, the incidence of surgical complications, and the surgical delay rate. Based on these data, we could conclude whether ICIs were safe when they were used before surgery. In these studies, investigators decided whether adverse events were treatment-related according to the study protocol and standard regulatory requirements. They also decided on the definition of surgical complications and surgical delay. After the analysis, the pooled results were 38.9% (95% CI, 23.6–64.0%), 19.0% (95% CI, 6.4–36.3%), 17.9% (95% CI, 6.8–29.1%) and 3.2% (95% CI, 0.4–8.7%), respectively. Detailed information can be found in Figure 3 and Figure S2.

Subgroup analysis
Due to the huge heterogeneity, we divided the studies into different groups to figure out the underlying reason. When we compared studies using only ICIs and those combining chemotherapy and ICIs, the difference was obvious. Generally, combination therapy obtained superior efficacy, reflected by the higher ORR (68.4% vs. 11.9%), MPR (64.1% vs. 23.6%) (Figure 4), and pCR (35.4% vs. 5.2%) (Figure S2). However, TRAE (57.3% vs. 31.1%) and high-grade TRAE (37.3% vs. 8.4%) increased. The differences between other outcomes were comparable.

When we changed the focus from the combination strategy of ICIs and chemotherapy to the type of ICIs, the same tendency appeared between PD-1 inhibitors and PD-L1 inhibitors. In terms of radiologic response and pathologic response, PD-1 inhibitors showed better performance compared with PD-L1 inhibitors. Pooled ORR, MPR (Figure 5), and pCR (Figure S3) for PD-1 inhibitor was 43.1% (95% CI, 23.0–63.3%), 50.5% (95% CI, 36.4–64.6%) and 26.8% (95% CI, 14.8–38.8%), separately. For PD-L1 inhibitor, outcome was 32.0% (95% CI, 0–64.1%), 29.5% (95% CI, 2.0–71.2%) and 13.9% (95% CI, 0–29.0%), respectively. As for safety, TRAE in the PD-1 inhibitor subgroup was 41.1%, and the other one was 17.6% (Figure 5). Additionally, the surgical complication was 25.8% in PD-1 inhibitor and 2.8% in PD-L1 inhibitor. However, 3–5 grade TRAE in PD-1 inhibitor (14.1%; 95% CI, 5.1–26.6%) showed an inferior performance compared with PD-L1 inhibitor (27.0%; 95% CI, 0–89.8%). The combined outcomes in the R0 rate, surgical complication, and surgical resection rate were similar between groups (Figure S3).

Exploratory analysis
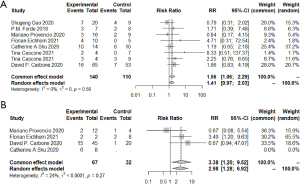
To further identify the possible biomarkers predicting prognosis, we conducted a meta-analysis of possible factors affecting pathologic response. First of all, the PD-L1 tumor proportion score (TPS) was a significant biomarker in advanced lung cancer. In this article, we explored whether PD-L1 TPS related to short-term outcomes, MPR, or pCR, which could be closely relevant to long-term prognosis in chemotherapy. Via R, we compared those with PD-L1 expression positive (≥1%) and those with PD-L1 expression negative (<1%) in participants with MPR. The combined RR of 8 trials was 1.56 (95% CI, 1.06–2.29) (Figure 6). When comparing PD-L1 high-expressing patients with PD-L1 low-expressing patients, the pooled RR was 3.38 (95% CI, 1.20–9.52) (Figure 6). We also considered those with low PD-L1 expression and those with PD-L1 negative expression. The average RR was 0.85 (95% CI, 0.25–2.87) (Figure S4). In terms of the relationship between radiologic response and pathologic response, except for one individual trial, individual RRs of the other 6 trials were in favor of patients with ORR (pooled ORR: 3.19; 95% CI, 2.17–4.69) (Figure 7). It indicated patients with ORR could get higher MPR than those without ORR. As for other possible factors such as smoking status, histological type, gender, and clinical stage, we found no significant results by meta-analysis of limited data (Figure S5). In particular, no difference was found in MPR between patients with stage III disease and those with early-stage tumors. Because data regarding stage I and IB were limited, we could not give relatively accurate results.
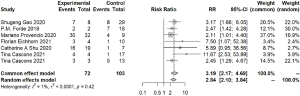
Similar results could be found when we compared those factors in patients with pCR (Figure S6). The average RR was 2.03 (95% CI, 1.15–3.58) when considering those with PD-L1 positive expression and those with PD-L1 negative expression. As to patients with radiologic responses and those without, the combined RR of 7 trials was 3.36 (95% CI, 1.89–5.98). No significant results were found when considering histological type or gender.
Analysis through meta-regression
Except for subgroup analysis, we also conducted meta-regression to define how many cycles were more recommended and when should operators do surgery after the first dose of ICIs. The analysis found no relationship between different cycles (P=0.16), the median duration of surgery (P=0.37), and MPR. Not median duration (P=0.09), cycles (P=0.02) had a relatively close relationship with pCR.
Study heterogeneity and risk of bias
We applied funnel plots to evaluate heterogeneity due to the huge values of I2. Results implied that the publication bias of the studies included was tolerable. Subgroup analysis also indicated the possible sources of bias.
Because all the trials included were phase 2 studies except CheckMate 816, most of which were non-RCTs. Thus, we utilized MINORS to make the assessment and found a low risk of bias (Figure S7).
Discussion
Our study did a relatively comprehensive update with 19 trials included. Compared with chemotherapy, immunotherapy gained better outcomes in pathologic response, surgical outcomes, and even adverse events. As to radiologic response, chemotherapy seemed to be better, the same as the previous meta-analysis (30). What’s more, we did abundant subgroup analysis and found the outstanding efficacy of combined therapy accompanied by worse adverse events as we discussed prior. It was finally proved that 3 cycles of nivolumab were preferred. When it comes to pathologic response, PD-L1 expression level may be the possible biomarker. Also, radiologic response reflected pathologic response to some extent.
Two meta-analyses evaluating the feasibility of neoadjuvant immunotherapy have been applied in resectable NSCLC (30,31). However, due to the limited data available, less than 10 trials were included. Different from our article, a study published in 2020 (31) reached only one conclusion that the safety and efficacy of neoadjuvant immunotherapy were supported owing to the relatively higher rate of pCR in several trials and lower pooled incidence of TRAE. However, in that article, few indicators were defined to measure effectiveness and safety due to a lack of data. Also, subgroup analysis didn’t compare single-drug immunotherapy and therapy combined ICIs and chemotherapy. Our research did a more comprehensive analysis. Considering the interference of chemotherapy, we divided all trials into two subgroups. According to our analysis, accompanied by better performance in radiologic response and pathologic response, the incidence of adverse events increased in the combined group. Furthermore, information extracted from 18 articles gave us a chance to conduct more detailed subgroup analyses and exploratory analyses. On the other hand, another article in 2021 (30) focused on short-term efficacy and surgical efficacy, lacking data on safety. Several trials gained a high incidence of TRAE, which could not be ignored. For example, in NADIM (17), a trial applying nivolumab and chemotherapy, 93.5% of participants had TRAE and 30.4% of participants suffered from 3–5 grade TRAE, which was considerably higher than that in neoadjuvant chemotherapy (32). Regardless of the numerous TRAE, our study showed the endurable pooled results in the incidence of surgical complication and delay no matter how the trials were grouped. Moreover, detailed subgroup analyses such as type of ICIs and treatment cycles were unable to conduct. The conclusion that the combined therapy was more recommended was consistent with ours to some extent. What is worth learning is that the article extracted data on neoadjuvant chemotherapy, giving a more straightforward comparison between chemotherapy and immunotherapy. Furthermore, possible biomarkers are lacking in discussion in the 2 articles mentioned. In our research, PD-L1 level and radiologic response were discussed for the first time. To sum up, our research updated from the previous 2 articles is worth conducting.
Choosing between PD-1 inhibitor and PD-L1 inhibitor was not analyzed in previous studies due to the deficiency of data. In our study, including 5 trials applying PD-L1 inhibitor, we found inferior performance. The rate of MPR gained 50.5% in the PD-1 inhibitor group whereas the PD-L1 inhibitor group gained only 29.5%. Interestingly, though the incidence of adverse events was higher the in PD-1 inhibitor group, the incidence of 3 to 5 grade TRAE was lower (14.1% vs. 27.0%), indicating feasibility when we applied PD-1 in clinical practice.
In previous trials exploring neoadjuvant chemotherapy, the researchers preferred to use MPR as their surrogate endpoint on the basis that histopathologic response related strongly to long-term OS (9,10). Most trials exploring the efficacy of neoadjuvant immunotherapy also tended to use MPR as their surrogate endpoint. Although pCR was considered to be a favorable prognostic factor (33), the number of patients who achieved pCR was pretty low, restricting its use in neoadjuvant chemotherapy (9,34). In NADIM, all patients with MPR or pCR stayed alive after 24 months of follow-up, supporting the possibility of using them as surrogate endpoints. Though most articles used MPR or pCR as a surrogate endpoint, the real relationship between pathologic response and prognosis requests more long-term follow-up data. In this article, we utilized MPR and pCR to evaluate prognosis.
PD-L1 level is a biomarker widely used in advanced NSCLC (35-37) and several clinical trials would like to explore its use of it in neoadjuvant immunotherapy (16,17,20,21). Unfortunately, controversial results were reached in these trials because individual cases with low PD-L1 expression reached surprising results as well. For example, in NEOMUN, researchers found positive relationships while a complete responder with a PD-L1 expression level of 1% existed. Herein, we analyzed data extracted from 8 studies and concluded that PD-L1 could be a potential biomarker predicting pathologic response in neoadjuvant immunotherapy. Specifically, PD-L1 positive patients achieved higher MPR than PD-L1 negative ones. Furthermore, in those with positive expression, high expression ones (TPS >50%) could be a more advantaged group.
On the other hand, the radiologic response has been a complicated indicator when using ICIs because of the pseudoprogression (38). In our study, after analyzing 7 trials involved, we found a significant difference that radiologic response could be another potential indicator of pathologic response in neoadjuvant immunotherapy. In NEOSTAR, researchers found positive relationships between radiologic response and pathologic response. Furthermore, because no pseudoprogression was found in NEOMUN, researchers concluded that computed tomography (CT) helped predict pathologic response. However, in chemotherapy, it was inconsistent that RECIST could be an incredible factor to predict OS or histopathologic response. Compared with ORR, pathologic response seemed more likely to be the possible predictor of prognosis (39). Moreover, in ChiCTR-OIC-17013726, researchers found a significant correlation between maximum standardized uptake value (SUVmax) reduction and pathologic response, giving credit to positron emission tomography (PET)-CT. On the other hand, about those who didn’t reach MPR or pCR, almost all the participants in ChiCTR-OIC-17013726 experiencing surgery while failing to achieve pathologic response tended to have lower SUVmax reduction (mostly <30% except one) while 13 patients reaching MPR measured had SUVmax reduction of more than 30% More controlled trials should be conducted to explore these conclusions. What is the role of PET-CT can be a possible direction for researchers to explore (40).
Tumor subtype, smoking status, and gender showed no significant difference in MPR. We got the same results in the tumor stage such as stage III. It may be different from some previous individual studies such as ChiCTR-OIC-17013726, which found higher MPR in squamous carcinoma. The limitation of meta-analysis and the limited data available could be the underlying explanation.
Except for MPR, we also used pCR for the evaluation, and the results reached were similarly the same.
We do have several limitations in this meta-analysis. Firstly, most of the studies involved were single-arm trials and some of them had only abstracts published at a recent conference. Consequently, the bias of these studies could be high. Missing data added to the difficulty of analysis. More RCTs should be included in the future. Secondly, the literature search may have language bias since no non-English language databases were searched. Thirdly, our study analyzed the trend between groups and we cannot provide an accurate statistical difference between comparison groups. Furthermore, long-term data was missing in our study for limited data. As a result, we gave more attention to MPR and pCR, which could be potential indicators reflecting prognosis. In addition, several new biomarkers were lack of data such as circulating tumor DNA (ctDNA) in peripheral blood or tumor mutational burden.
Conclusions
In conclusion, our meta-analysis confirms previous studies supporting the efficacy and safety of immunotherapy compared with traditional chemotherapy. Moreover, combination therapy is preferred in efficacy and its safety is acceptable. Except for the conclusion above with more trials involved, we also consumed that the PD-1 inhibitor was better. Regarding potential biomarkers, PD-L1 expression can be a strong factor. In our study, the radiologic response also owns a close relationship with MPR. The ongoing trials may provide detailed results in the future. More analysis should be conducted to support its wider application in clinical practice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-88/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-88/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-88/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest 2003;123:2096-103. [Crossref] [PubMed]
- Boyd JA, Hubbs JL, Kim DW, et al. Timing of local and distant failure in resected lung cancer: implications for reported rates of local failure. J Thorac Oncol 2010;5:211-4. [Crossref] [PubMed]
- Song WA, Zhou NK, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. J Thorac Oncol 2010;5:510-6. [Crossref] [PubMed]
- Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. [Crossref] [PubMed]
- Borghaei H, Gettinger S, Vokes EE, et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol 2021;39:723-33. [Crossref] [PubMed]
- Gao S, Li N, Gao S, et al. Two-year follow-up of single PD-1 blockade in neoadjuvant resectable NSCLC. J Clin Oncol 2021;39:abstr 8522.
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Yang CJ, McSherry F, Mayne NR, et al. Surgical Outcomes After Neoadjuvant Chemotherapy and Ipilimumab for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:924-9. [Crossref] [PubMed]
- Bar J, Urban D, Ofek E, et al. Neoadjuvant pembrolizumab (Pembro) for early stage non-small cell lung cancer (NSCLC): Updated report of a phase I study, MK3475-223. J Clin Oncol 2019;37:abstr 8534.
- Wislez M, Mazieres J, Lavole A, et al. 1214O Neoadjuvant durvalumab in resectable non-small cell lung cancer (NSCLC): Preliminary results from a multicenter study (IFCT-1601 IONESCO). Ann Oncol 2020;31:S794. [Crossref]
- Ready N, Tong B, Clarke J, et al. P2. 04-89 Neoadjuvant pembrolizumab in early stage non-small cell lung cancer (NSCLC): toxicity, efficacy, and surgical outcomes. J Thorac Oncol 2019;14:S745.
- Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020;15:816-26. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. [Crossref] [PubMed]
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. [Crossref] [PubMed]
- Zinner R, Axelrod R, Solomides CC, et al. Neoadjuvant nivolumab (N) plus cisplatin (C)/pemetrexed (P) or cisplatin/gemcitabine (G) in resectable NSCLC. J Clin Oncol 2020;38:abstr 9051.
- Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504-14. [Crossref] [PubMed]
- Eichhorn F, Klotz LV, Kriegsmann M, et al. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: First clinical experience. Lung Cancer 2021;153:150-7. [Crossref] [PubMed]
- Besse B, Adam J, Cozic N, et al. 1215O-SC Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): Results from the phase II PRINCEPS trial. Ann Oncol 2020;31:S794-5. [Crossref]
- Rothschild SI, Zippelius A, Eboulet EI, et al. SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial. J Clin Oncol 2021;39:2872-80. [Crossref] [PubMed]
- Spicer J, Wang C, Tanaka F, et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO)+ platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J Clin Oncol 2021;39:abstr 8503.
- Zhang Y, Zeng L, Zhang X, et al. P15. 02 Toripalimab and Platinum-Doublet Chemotherapy as Neoadjuvant Therapy for Potentially Resectable Non-Small Cell Lung Cancer. J Thorac Oncol 2021;16:S1014-5.
- Zhao Z, Chen S, Qi H, et al. Phase II trial of toripalimab plus chemotherapy as neoadjuvant treatment in resectable stage III non-small cell lung cancer (NeoTPD01 Study). J Clin Oncol 2021;39:abstr 8541.
- Kwiatkowski DJ, Rusch VW, Chaft JE, et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol 2019;37:abstr 8503.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Zhang C, Hong HZ, Wu YL, et al. Short-term outcome of neoadjuvant immunotherapy and chemotherapy in non-small cell lung cancer: A systematic review and meta-analysis. JTCVS Open 2021;8:588-607. [Crossref] [PubMed]
- Jia XH, Xu H, Geng LY, et al. Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: A meta-analysis. Lung Cancer 2020;147:143-53. [Crossref] [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Mouillet G, Monnet E, Milleron B, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol 2012;7:841-9. [Crossref] [PubMed]
- Blumenthal GM, Bunn PA Jr, Chaft JE, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol 2018;13:1818-31. [Crossref] [PubMed]
- Brody R, Zhang Y, Ballas M, et al. PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017;112:200-15. [Crossref] [PubMed]
- Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542-51. [Crossref] [PubMed]
- Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275-87. [Crossref] [PubMed]
- Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol 2015;33:3541-3. [Crossref] [PubMed]
- William WN Jr, Pataer A, Kalhor N, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2013;8:222-8. [Crossref] [PubMed]
- Chetan MR, Gleeson FV. Radiomics in predicting treatment response in non-small-cell lung cancer: current status, challenges and future perspectives. Eur Radiol 2021;31:1049-58. [Crossref] [PubMed]
Cite this article as: Xu K, Ni J, Wu X, Xie J, Chen M, Zhang F, Liu H, Zhan P, Lv T, Song Y. Efficacy and safety of neoadjuvant immunotherapy in non-small cell lung cancer: a systematic review and meta-analysis. AME Med J 2022;7:35.


