
A clinical practice review of the surgical approach for distal esophageal tumors
Introduction
Background
Esophagectomy for cancer is generally more complex than resection of most other cancer types. The esophagus’ course through the neck, chest, and abdomen necessitates access to at least two body cavities to achieve both cancer resection and gastrointestinal (GI) reconstruction. Several approaches to esophagectomy exist, but most require multiple incisions or patient repositioning, surgical manipulation of not only the esophagus but also the stomach or another GI organ as a conduit to restore GI continuity, and mobilization of the conduit into the thorax, which risks compromise of its blood supply. The morbidity and mortality associated with esophagectomy has improved over time but is still higher than most surgical procedures (1-3).
The most appropriate option for a particular patient depends on specific characteristics of the patient and their cancer, as well as the surgeon who performs the surgery and the institution where they receive their care. Considering that the prognosis of esophageal cancer is relatively poor even if diagnosed at an early stage, and even when combined with other therapeutic modalities (4), the most crucial aspects of esophagectomy for cancer are minimizing treatment morbidity while optimizing cancer outcomes. In addition, ultimate quality of life is also very important in terms of allowing a patient to have normal post-operative eating and swallowing.
Rationale and knowledge gap
The rationale for this review was to provide a practical, clinically-relevant overview of the factors at play when selecting a surgical approach for a patient with a distal esophageal tumor. Prior reviews have focused on the non-surgical aspects of esophageal cancer (5), or were published prior to the widespread adoption of minimally-invasive esophageal surgery (6). We hope this review will provide a current, useful overview for both surgeons and non-surgeon clinicians as they care for patients with esophageal cancer before and after surgical resection.
Objective
The objective of this review is to provide a brief outline of the epidemiology, diagnosis, staging, and treatment of tumors of the distal esophagus, followed by a detailed comparison of the various surgical approaches to esophagectomy, and an overview of outcomes and complications.
Distal esophageal tumors
Epidemiology
Esophageal cancer is relatively uncommon, representing only 1% of newly diagnosed cancers (7,8). Globally, esophageal cancer is the 7th most common cancer, with over 500,000 new cases diagnosed annually, and approximately the same number of cancer deaths, underscoring the low survival rate (9). The incidence is increasing in the United States, particularly among white males, although the disease is disproportionately more common among black males (4-10) Adenocarcinoma is the most common histology in the United States, followed closely by squamous cell carcinoma (7,11). Risk factors for adenocarcinoma include smoking, male sex, obesity, reflux disease, Barrett’s esophagus, and radiation, while alcohol, tobacco use, achalasia, and caustic ingestion are associated with squamous cell carcinoma (7). The majority of adenocarcinomas occur in the distal esophagus while the location of squamous cell cancers is more evenly distributed in the middle and distal third; thus the distal esophagus is the most common location of esophageal cancer overall (7).
Diagnosis and staging
Patients often present with dysphagia and/or weight loss, prompting evaluation with imaging and endoscopy (12). An upper GI may show narrowing or ulceration. Endoscopy will identify a mucosal-based mass that can be biopsied to obtain a tissue diagnosis. It is important to note the anatomic location of the tumor, including distance from the gastroesophageal junction, areas of Barrett’s esophagus that will require resection, and careful evaluation of the stomach to determine gastric involvement (and therefore suitability of the stomach for use as a conduit). Specifically, tumors centered around the lower esophagus that extend to the junction (Siewert Type I) and centered around the cardia, defined as 1 cm above to 2 cm below the gastroesophageal junction (Siewert Type II) are treated as esophageal cancers, and if resectable, will be treated with esophagectomy. Siewert Type III, defined as arising from 2 to 5 cm distal to the junction are treated as gastric cancers but typically require some resection of the distal esophagus during gastrectomy to ensure an adequate margin (13). To complete clinical staging, computed tomography (CT) scan of the chest/abdomen/pelvis, usually with positron emission tomography (PET), is used to determine whether metastatic disease is present (M stage), and endoscopic ultrasound is used to determine the depth of invasion (T stage) and identify suspicious regional lymph nodes (N stage) (14). Although not necessary for oncologic work-up, cardiopulmonary evaluation with pulmonary function testing or cardiology evaluation can assist in determining a patient’s ability to ultimately tolerate surgery the patient is elderly or has significant comorbid conditions.
Treatment
Treatment approach is determined by clinical stage, which can be thought of more broadly in three categories: (I) early-stage superficial cancers, (II) cancers that are locally advanced with locoregional disease but without distant disease, and (III) cancers with distant metastasis (8). Patients with early-stage superficial disease proceed directly to resection (11). Local therapies, such as endoscopic mucosal resection, ablation, cryotherapy, and photodynamic therapy can be considered for patients with T1a tumors, in order to avoid the morbidity associated with esophagectomy (15,16) Pathology must confirm negative margins and endoscopic surveillance is required. Patients with tumors involving the submucosa (T1b) who are medically fit for surgery should be treated with esophagectomy without induction therapy (11). Classifying tumors that involve the muscularis (T2) but without evidence of nodal involvement (N0) as early stage versus locally advanced can be difficult, and these patients are often ultimately up-staged or down-staged pathologically (17). Our approach to patients with cT2N0 tumors is generally to proceed with esophagectomy when the tumor is incidentally found on endoscopy or for work up for mild symptoms, and to begin with induction therapy when patients present with symptoms suggestive of a bulky, locally advanced tumor. Those with locally advanced disease due to lymph node involvement (cN1) or with bulky tumors (T3, invading the adventitia of the esophagus) should be treated with induction therapy (neoadjuvant chemotherapy with or without radiation), followed by restaging imaging to determine whether they are candidates for surgical resection (11,18). Patients with metastatic disease do not typically benefit from surgical resection, and are referred for palliative therapy, which may involve chemotherapy or symptom-directed local therapy such as radiation, endoscopic dilation or stenting (19).
Surgical techniques
General and oncologic considerations
Esophagectomy and restoration of GI continuity is technically complex, and although reported mortality rates have significantly decreased over time, the associated morbidity remains high (1-3). Given that the esophagus begins in the neck, traverses the chest in the posterior mediastinum, and ends in the abdomen, resection requires access to at least two, and sometimes all three of these spaces via separate incisions. Adjacent structures that may be damaged during resection include the trachea and mainstem bronchi, recurrent laryngeal nerves, aorta, thoracic duct, and spleen.
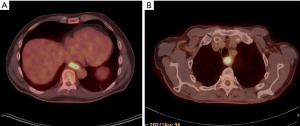
Selection of surgical approach is multifactorial. Considerations include the patient’s comorbidities, especially pulmonary function, as well as prior surgical history, and surgeon/institutional preference. However, the selected technique must achieve the primary oncologic goals of obtaining both a complete resection with negative margins and an adequate lymphadenectomy. A detailed understanding of the location and extent of the tumor is critical in order to obtain 5-cm proximal and distal margins, though most esophagectomy techniques can achieve these margins for distal tumors (Figure 1) (20).
However, another aspect of esophageal resection for cancer whose oncologic importance has only somewhat recently been recognized is the extent of lymphadenectomy performed, which has critical implications for prognosis and the use of post-operative therapy. Lymphadenectomy is important to survival regardless of whether patients have received induction therapy, though the lymph node harvest can be decreased when patients have gotten induction therapy (21-24). Data from the Worldwide Esophageal Cancer Collaboration (WECC) demonstrated that the optimum lymphadenectomy for pN0 patients not treated with induction therapy was 10 to 12 nodes for pT1 tumors, 15 to 22 nodes for pT2 tumors, and 31 to 42 nodes for pT3/T4 tumors, depending on histopathologic cell type (23). The optimum lymphadenectomy for patients with pN+M0 cancers where 1 to 6 nodes were positive was 10 nodes for pT1 tumors, 15 nodes for pT2 tumors, and 29 to 50 nodes for pT3/T4 tumors (23). Interestingly, and perhaps not intuitively appreciated, a more aggressive lymph node resection is necessary to accurately define pN+ status for smaller and earlier stage tumors, as deeper invading, longer, and poorly differentiated esophageal cancers have more positive nodes and so pN+ status can be more easily demonstrated with resection of fewer nodes, while superficial, shorter, and well-differentiated cancers require a more extensive lymphadenectomy to accurately define pN+ status (25). Table 1 lists various techniques for esophagectomy, which are described in detail below, including how they perform in regards to margins, lymph node resection, and complications.
Table 1
| Factors influencing selection of approach | Ivor Lewis | Three-incision (McKeown) | Transhiatal | Left thoraco-abdominal |
|---|---|---|---|---|
| Incisions | Thoracic and abdominal | Cervical, thoracic and abdominal | Cervical and abdominal | Large thoraco-abdominal incision |
| Extent of proximal margin | Potentially limited | Maximal | Maximal | Most limited |
| Node dissection | Complete | Complete | Limited | Complete |
| Location of anastomosis | Thoracic | Cervical | Cervical | Thoracic |
| Advantages | ↓ Risk of recurrent laryngeal nerve injury | Access to entire esophagus | ↓ Pulmonary complications | Useful in pts with abdominal adhesions/multiple prior surgeries |
| ↓ Postoperative reflux symptoms | ↓ Postoperative reflux symptoms | |||
| No repositioning required | ||||
| Disadvantages | ↑ Postoperative reflux symptoms | ↑ Risk of recurrent laryngeal nerve injury | ↑ Risk of recurrent laryngeal nerve injury | ↑ Postoperative reflux symptoms |
| May not address Barrett’s extending proximally | Blunt thoracic dissection without visualization | ↑ Rate of + margins |
↑, increased; ↓, decreased.
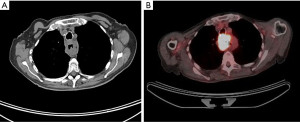
Some aspects are common to all approaches. Before proceeding to the operating room, it is important to evaluate and optimize any comorbid conditions, as well as the patient’s nutritional status. Patient who are malnourished due to intolerance of oral intake may benefit from a period of jejunostomy tube feeding preoperatively. Prior to beginning the esophagectomy, endoscopy should be repeated in the operating room to confirm findings reported during the patient’s initial workup. For proximal and mid-esophageal tumors, bronchoscopy is needed to exclude invasion of the airway (Figure 2). Placement of an orogastric or nasogastric tube helps to facilitate manipulation of the esophagus intraoperatively. Surgeons may routinely or selectively place jejunostomy tubes to assist in postoperative feeding; our practice is to do so in all cases. Tubularization of the stomach is the most common method used for reconstruction, and results in good functional outcomes (26). As the vagus nerve is transected during esophagectomy, surgeons may perform pyloromyotomy, pyloroplasty, or Botox injection to improve conduit emptying, although this has not been shown to improve functional outcomes when reviewed retrospectively (27). In patients in whom the stomach is not appropriate for use, for example in patients with a history of gastric resection, weight loss surgery, gastroparesis, or tumors extending distally in to the stomach, interposition of a segment of jejunum or colon may be used (28-30).
Ivor Lewis esophagectomy
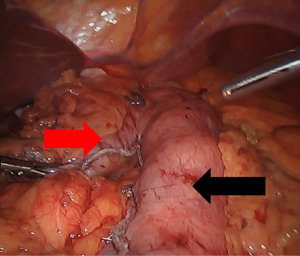
The Ivor Lewis esophagectomy is the most common approach for tumors of the distal esophagus, and is performed via the right thorax and abdomen (31). First, the peritoneum is accessed and the stomach is mobilized with preservation of the right gastroepiploic artery that serves as the conduit’s blood supply. The left gastric artery is ligated and divided, and then using fires of a linear stapler, the lesser curve of the stomach is excised, creating a gastric tube 4–6 cm in width (Figure 3). Care is taken to maintain a straight staple line to avoid a “cork-screw” effect on the conduit. Then the patient is repositioned in the left lateral decubitus position, and the right chest is accessed. With the assistance of single-lung ventilation and division of the azygous vein, the esophagus is mobilized to from the hiatus to several centimeters above the carina, and a complete lymphadenectomy is performed. After proximal division of the esophagus, the conduit is brought up in to the chest, and divided distally. The anastomosis will lie in the posterior mediastinum, and can be handsewn or constructed with the help of a circular or linear stapling device (Figure 4). A nasogastric tube is placed under direct vision across the anastomosis, and a chest tube is placed for drainage.
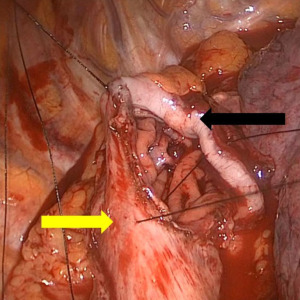
This approach is useful for patients with distal esophageal tumors without significant proximal extension of Barrett’s esophagus, as the proximal extent of resection is typically about 25–30 cm from the incisors.
Three-incision esophagectomy (McKeown)
The three-incision esophagectomy, also called McKeown esophagectomy, begins in the right chest with the patient in left lateral decubitus position (32). The esophagus is fully mobilized from the hiatus to the thoracic inlet, and complete lymph node dissection is performed. This is done with single-lung ventilation and division of the azygous vein. During proximal dissection, care must be taken to avoid injury to the airway and recurrent laryngeal nerve. A chest tube is placed and the incisions are closed. The patient is then turned to the supine position with a shoulder roll in place and the chin turned to the right to expose the left neck. The abdomen is entered and the conduit is prepared in the same way as described above for Ivor Lewis esophagectomy. However, as the gastric conduit must reach the neck, additional mobilization maneuvers, such as full Kocherization of the duodenum, and ligation of the right gastric artery, may be required. After the esophagus has been fully dissected in the chest and the conduit prepared in the abdomen, a left neck incision is made and the cervical esophagus is mobilized. Care is taken to preserve the left recurrent laryngeal nerve. After proximal transection, a stitch or Penrose drain is affixed to the esophagus (this will help guide the conduit up through the mediastinum subsequently), and it is pulled down in to the abdomen. The distal margin is selected and the specimen excised, and the gastric conduit is pulled back up to the neck using the Penrose, taking care not to twist the conduit. The cervical anastomosis can be handsewn or stapled, and the nasogastric tube is advanced through the anastomosis under direct vision. A soft drain is often placed and the skin is usually stapled to facilitate partial opening in the event of a leak or wound infection.
The advantages of McKeown esophagectomy are that this approach provides excellent margins for all tumors, including those with proximal extension, and provides excellent access to all nodal stations. It is also associated with less symptomatic gastroesophageal reflux than Ivor Lewis esophagectomy because less esophageal mucosa remains (33). The benefits of cervical, as opposed to mediastinal location of the anastomosis is somewhat controversial (34-36). Leaks may be more common, given the longer length of conduit required to reach the neck, but there may be less associated morbidity in the event of a leak, due to the absence of mediastinal contamination.
Transhiatal esophagectomy
Transhiatal esophagectomy is performed by accessing the abdomen and left neck (37). As in a three-field esophagectomy, the gastric conduit is prepared via the abdomen (described above). Then the surgeon passes a hand through the hiatus to bluntly dissect the thoracic esophagus free from surrounding structures, taking care to avoid injury to the recurrent laryngeal nerve and mediastinal vasculature. A left neck incision is made, and the conduit can be pulled up to create a cervical anastomosis, in the same manner as in the three-field technique.
The major advantage of transhiatal esophagectomy is that no thoracic incision is required, thus minimizing pulmonary complications and associated morbidity. There is also no need to reposition the patient intraoperatively, nor is single-lung ventilation required. However, because the thoracic portion of the dissection is performed bluntly, a complete nodal dissection is not possible. Given this limitation, utilization of the transhiatal approach has decreased over time (36). Additionally, there is added risk of mediastinal hemorrhage and injury to thoracic structures (38). Significant intraoperative hemorrhage during blunt dissection may require expeditious right or left thoracotomy to expose and repair the injury. The transhiatal approach may be well-suited for patients with Barrett’s and high-grade dysplasia not amenable or refractory to endoscopic therapies, where a significant proximal margin is needed but complete lymphadenectomy is less critical.
Left thoracoabdominal esophagectomy
The left thoracoabdominal approach can be used to approach tumors of the distal esophagus that are located at least 30–35 cm from the incisors. The patient is positioned in a modified right lateral decubitus position, with the left hip tilted back to allow exposure of the abdomen. A large somewhat curved incision from the 7th intercostal space, across the costal margin, and curving towards the abdominal midline is generally utilized. Single lung ventilation is used for exposure, and the diaphragm is taken down circumferentially to allow access to the distal esophagus and proximal stomach for mobilization, resection, and anastomosis. A chest tube is placed and the diaphragm must be reapproximated.
This approach is generally reserved for patients with prior abdominal operations in whom approaching the stomach and hiatus via midline laparotomy is expected to be prohibitively difficult. Although only a single incision is required, it is often associated with significant postoperative pain which can predispose patients to pulmonary complications, particularly those that are elderly or frail. The extent to which the stomach and esophagus can be mobilized from this approach is limited, resulting in higher risk of incomplete resection (39). Symptomatic gastroesophageal reflux is common given the low thoracic location of the anastomosis and greater amount of residual native esophagus compared to other approaches that allow a more extensive resection.
Minimally-invasive esophagectomy
The thoracic and/or abdominal portions of the different approaches to esophagectomy are increasingly being performed minimally-invasively, using thoracoscopy, laparoscopy, or robotic-assisted surgery. Use of minimally-invasive approaches does not compromise oncologic outcomes nor does it increase mortality (40,41). While minimally-invasive techniques may require longer operating times, they may also reduce morbidity, specifically pulmonary complications (by obviating the need for a thoracotomy) and recovery time (42).
As has been shown for lung cancer surgery, a minimally-invasive approach may provide the most significant potential benefit to higher risk patients who are elderly, have a history of significant pulmonary disease, are frail, or have impaired pulmonary function. If a patient is deemed as having prohibitive risks for surgery by a surgeon who does not use minimally-invasive techniques, another opinion at a high-volume center that performs minimally invasive surgery should be considered before a patient ultimately denied what is likely a critical modality for potential cure of their cancer.
Outcomes and complications
Mortality
Esophagectomy continues to be a high-risk operation; historic mortality rates were reported around 10% (43,44). With improvements in surgical technique, anesthesia, critical care, and interventional radiology and advanced endoscopy for management of complications, recent data indicate a mortality rate centered around 3%, with some high-volume centers reporting mortality rates as low as 1% (1-3,45,46). Factors associated with perioperative mortality include higher Charlson comorbidity index, age, kidney disease, diabetes, history of alcohol abuse, impaired preoperative functional status, ascites, and intraoperative transfusion (3,46,47). Increased mortality is also associated with undergoing surgery at a lower volume center, which has been variably previously defined as <12 esophagectomies per year, or <7 per surgeon or <20 per center per year (48-50). Having surgery at a higher volume center is also associated with improved long-term survival compared to lower volume centers (51). More recent literature has reinforced the impact of surgeon and hospital volumes on outcomes, and the best definition for a “high-volume” center continue to evolve (51,52).
Anastomotic leak
Anastomotic leak is a significant source of morbidity after esophagectomy and continues to occur in 10–15% of patients (1,34,35,46,47). Patients may present with fever, leukocytosis, change in character of drainage from surgical tubes, or sepsis. Depending on the surgical approach, the anastomosis will either lie in the neck (three-field and transhiatal) or in the mediastinum (Ivor Lewis and thoracoabdominal). Traditionally, cervical leaks were thought to have less associated morbidity, as they are contained in the soft tissue of the neck and source control can be obtained by allowing open drainage through the neck incision (46). Thoracic leaks may contaminate the mediastinum and pleural space, leading to mediastinitis, sepsis, and greater difficulty obtaining source control. However, cervical anastomoses may be associated with a higher overall leak rate, perhaps due to the longer conduit length required, which can increase the risk of ischemia or tension on the anastomosis. A randomized trial of cervical versus thoracic anastomoses in minimally-invasive esophagectomy examined rate of leak as the primary outcome, and indeed found lower leak rates in the thoracic anastomosis group (12% versus 34%), as well as fewer severe complications, a lower rate of recurrent laryngeal nerve injury, and better functional outcomes (53).
The management of a leak depends on the severity, and to some extent the location. Leaks that are well-drained may be managed by keeping the patient nil per os (NPO), antibiotics and antifungals, and jejunostomy tube feeding. Others may require placement of additional drainage tubes (for thoracic leaks) or wound exploration (for cervical leaks). The use of endoscopic stenting of the esophagus to exclude the defect is increasing, although this can be difficult for a cervical leak where a stent may not be well tolerated if the proximal extent causes pharyngeal symptoms (54). Severe leaks will require reoperation to attempt repair or to perform a diverting esophagostomy. Conduit necrosis is a dreaded complication with high mortality that requires prompt debridement of the necrotic tissue and diversion, followed by a subsequent attempt at GI reconstruction with a different conduit choice when the patient has recovered (55).
Strictures may occur at the anastomosis, possibly as a result of a leak and/or ischemia. Patients with symptoms of dysphagia or regurgitation due to strictures can be managed with endoscopic dilation (56).
Respiratory complications
Patients undergoing esophagectomy are at increased risk of respiratory complications such as aspiration and pneumonia from impaired clearance of secretions or impaired conduit emptying (1,2). In fact, post-operative pneumonia is a significant risk factor for peri-operative death (46). Pain control, coughing exercises and pulmonary toilet, as well as early mobilization are important aspects of postoperative management.
Surgical technique selection also can influence the risk of post-operative respiratory complications. Thoracotomy probably increases the risk of pneumonia, and can be avoided by transhiatal approach or the use of thoracoscopy for the chest portion of Ivor Lewis or three-field esophagectomy (42). Impaired conduit emptying may be caused by redundant stomach pulled in to the chest, and patients are at risk of aspiration, especially when lying down. Recurrent laryngeal nerve injury during esophagectomy can cause vocal cord paralysis (57). Transhiatal and three-field approaches increase the risk of this complication four-fold (8,53). The paralyzed vocal cord should be medialized via injection if the patients has an impaired cough, as this decreases the risk of pneumonia (57).
Selection of approach
All of the surgical approaches for distal esophageal cancer have advantages and disadvantages (Table 1). In general, avoiding a thoracotomy can reduce pulmonary morbidity. Performing a cervical anastomosis may maximize the margin obtained, but has an associated risk of recurrent laryngeal nerve injury and subsequent respiratory complications. A cervical anastomosis may have a higher leak rate, but a cervical leak may also be easier to manage with less morbidity than an intra-thoracic leak. Minimally-invasive approaches can reduce surgical trauma and improve respiratory morbidity, but may have higher leak rates early in a surgeon’s experience. A trans-hiatal approach can allow a long margin and reduce some operative morbidity, but does involve some specific intra-operative risks and more importantly has a limited dissection of intra-thoracic lymph nodes. Our preference for treating distal esophageal tumors is to perform a minimally-invasive Ivor Lewis esophagectomy with laparoscopy and thoracoscopy, assuming that an anastomosis at about 25 cm will provide at least a 5-cm margin. However, each surgeon should choose the technique that allows them to perform an adequate and appropriate oncologic procedure while minimizing peri-operative death and complications.
Strengths and limitations
The strengths of our review include the comprehensive nature of the discussion of the various approaches to esophagectomy, and their relevant indications and complications. The limitation of our work is that it is not a systematic review of all literature regarding esophagectomy, but rather a practical review of the considerations at play when selecting the surgical approach for a specific clinical scenario.
Conclusions
The different surgical approaches to esophagectomy for tumors of the distal esophagus have some unique advantages and disadvantages. Certain patients will require a specific approach, while others may be suitable for multiple approaches; in such cases, surgeon and institutional preference will influence the choice of esophagectomy.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-53/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-53/coif). MFB serves as an unpaid editorial board member of AME Medical Journal from March 2019 to July 2023. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu JF, Wang QZ, Ping YM, et al. Complications after esophagectomy for cancer: 53-year experience with 20,796 patients. World J Surg 2008;32:395-400. [Crossref] [PubMed]
- Law S, Wong KH, Kwok KF, et al. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg 2004;240:791-800. [Crossref] [PubMed]
- Ra J, Paulson EC, Kucharczuk J, et al. Postoperative mortality after esophagectomy for cancer: development of a preoperative risk prediction model. Ann Surg Oncol 2008;15:1577-84. [Crossref] [PubMed]
- Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2018. Bethesda, MD: National Cancer Institute. https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021.
- Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014;6:112-20. [Crossref] [PubMed]
- Koshy M, Esiashvilli N, Landry JC, et al. Multiple management modalities in esophageal cancer: epidemiology, presentation and progression, work-up, and surgical approaches. Oncologist 2004;9:137-46. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Berry MF. Chapter 43: Esophageal Tumors and Injury. In: Dimick JB. Mulholland and Greenfield’s Surgery: Scientific Principles & Practice. 7th edition. Lippincott Williams & Wilkins, 2021.
- Huang J, Koulaouzidis A, Marlicz W, et al. Global Burden, Risk Factors, and Trends of Esophageal Cancer: An Analysis of Cancer Registries from 48 Countries. Cancers (Basel) 2021.
- Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049-53.
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562-72; discussion 572-3. [Crossref] [PubMed]
- Rüdiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000;232:353-61. [Crossref] [PubMed]
- Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis 2014;6:S289-97. [Crossref] [PubMed]
- Berry MF, Zeyer-Brunner J, Castleberry AW, et al. Treatment modalities for T1N0 esophageal cancers: a comparative analysis of local therapy versus surgical resection. J Thorac Oncol 2013;8:796-802. [Crossref] [PubMed]
- Worrell S, DeMeester SR. Endoscopic Resection and Ablation for Early-Stage Esophageal Cancer. Thorac Surg Clin 2016;26:173-6. [Crossref] [PubMed]
- Crabtree TD, Kosinski AS, Puri V, et al. Evaluation of the reliability of clinical staging of T2 N0 esophageal cancer: a review of the Society of Thoracic Surgeons database. Ann Thorac Surg 2013;96:382-90. [Crossref] [PubMed]
- Speicher PJ, Wang X, Englum BR, et al. Induction chemoradiation therapy prior to esophagectomy is associated with superior long-term survival for esophageal cancer. Dis Esophagus 2015;28:788-96. [Crossref] [PubMed]
- Ramakrishnaiah VP, Malage S, Sreenath GS, et al. Palliation of Dysphagia in Carcinoma Esophagus. Clin Med Insights Gastroenterol 2016;9:11-23. [Crossref] [PubMed]
- Barbour AP, Rizk NP, Gonen M, et al. Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg 2007;246:1-8. [Crossref] [PubMed]
- Raja S, Rice TW, Murthy SC, et al. Value of Lymphadenectomy in Patients Receiving Neoadjuvant Therapy for Esophageal Adenocarcinoma. Ann Surg 2021;274:e320-7. [Crossref] [PubMed]
- Stiles BM, Nasar A, Mirza FA, et al. Worldwide Oesophageal Cancer Collaboration guidelines for lymphadenectomy predict survival following neoadjuvant therapy. Eur J Cardiothorac Surg 2012;42:659-64. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Hanna JM, Erhunmwunsee L, Berry M, et al. The prognostic importance of the number of dissected lymph nodes after induction chemoradiotherapy for esophageal cancer. Ann Thorac Surg 2015;99:265-9. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Hofstetter WL, et al. Esophageal Cancer: Associations With (pN+) Lymph Node Metastases. Ann Surg 2017;265:122-9. [Crossref] [PubMed]
- Enestvedt CK, Perry KA, Kim C, et al. Trends in the management of esophageal carcinoma based on provider volume: treatment practices of 618 esophageal surgeons. Dis Esophagus 2010;23:136-44. [Crossref] [PubMed]
- Urschel JD, Blewett CJ, Young JE, et al. Pyloric drainage (pyloroplasty) or no drainage in gastric reconstruction after esophagectomy: a meta-analysis of randomized controlled trials. Dig Surg 2002;19:160-4. [Crossref] [PubMed]
- Cerfolio RJ, Allen MS, Deschamps C, et al. Esophageal replacement by colon interposition. Ann Thorac Surg 1995;59:1382-4. [Crossref] [PubMed]
- Kolh P, Honore P, Degauque C, et al. Early stage results after oesophageal resection for malignancy - colon interposition vs. gastric pull-up. Eur J Cardiothorac Surg 2000;18:293-300. [Crossref] [PubMed]
- Luan A, Hunter CL, Crowe CS, et al. Comparison of Outcomes of Total Esophageal Reconstruction With Supercharged Jejunal Flap, Colonic Interposition, and Gastric Pull-up. Ann Plast Surg 2018;80:S274-8. [Crossref] [PubMed]
- Harrington C, Molena D. Minimally invasive Ivor Lewis esophagectomy in 10 steps. JTCVS Tech 2021;10:489-94. [Crossref] [PubMed]
- D'Amico TA. Mckeown esophagogastrectomy. J Thorac Dis 2014;6:S322-4. [Crossref] [PubMed]
- D'Amico TA. Surgery for esophageal cancer. Gastrointest Cancer Res 2008;2:S6-9.
- Martin LW, Swisher SG, Hofstetter W, et al. Intrathoracic leaks following esophagectomy are no longer associated with increased mortality. Ann Surg 2005;242:392-9; discussion 399-402. [Crossref] [PubMed]
- Verstegen MHP, Slaman AE, Klarenbeek BR, et al. Outcomes of Patients with Anastomotic Leakage After Transhiatal, McKeown or Ivor Lewis Esophagectomy: A Nationwide Cohort Study. World J Surg 2021;45:3341-9. [Crossref] [PubMed]
- Schlottmann F, Strassle PD, Patti MG. Transhiatal vs. Transthoracic Esophagectomy: A NSQIP Analysis of Postoperative Outcomes and Risk Factors for Morbidity. J Gastrointest Surg 2017;21:1757-63. [Crossref] [PubMed]
- Orringer MB. Transhiatal Esophagectomy: How I Teach It. Ann Thorac Surg 2016;102:1432-7. [Crossref] [PubMed]
- Parekh K, Iannettoni MD. Complications of esophageal resection and reconstruction. Semin Thorac Cardiovasc Surg 2007;19:79-88. [Crossref] [PubMed]
- Forshaw MJ, Gossage JA, Ockrim J, et al. Left thoracoabdominal esophagogastrectomy: still a valid operation for carcinoma of the distal esophagus and esophagogastric junction. Dis Esophagus 2006;19:340-5. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Yang CJ, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Sihag S, Kosinski AS, Gaissert HA, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Comparison of Early Surgical Outcomes From The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101:1281-8; discussion 1288-9. [Crossref] [PubMed]
- Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75:217-22; discussion 222. [Crossref] [PubMed]
- Connors RC, Reuben BC, Neumayer LA, et al. Comparing outcomes after transthoracic and transhiatal esophagectomy: a 5-year prospective cohort of 17,395 patients. J Am Coll Surg 2007;205:735-40. [Crossref] [PubMed]
- Leigh Y, Goldacre M, McCulloch P. Surgical specialty, surgical unit volume and mortality after oesophageal cancer surgery. Eur J Surg Oncol 2009;35:820-5. [Crossref] [PubMed]
- Atkins BZ, Shah AS, Hutcheson KA, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg 2004;78:1170-6; discussion 1170-6. [Crossref] [PubMed]
- Raymond DP, Seder CW, Wright CD, et al. Predictors of Major Morbidity or Mortality After Resection for Esophageal Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk Adjustment Model. Ann Thorac Surg 2016;102:207-14. [Crossref] [PubMed]
- Dimick JB, Wainess RM, Upchurch GR Jr, et al. National trends in outcomes for esophageal resection. Ann Thorac Surg 2005;79:212-6; discussion 217-8. [Crossref] [PubMed]
- Dolan D, White A, Lee DN, et al. Short and Long-term Outcomes Among High-Volume vs Low-Volume Esophagectomy Surgeons at a High-Volume Center. Semin Thorac Cardiovasc Surg 2022;34:1340-50. [Crossref] [PubMed]
- Clark JM, Cooke DT, Hashimi H, et al. Do the 2018 Leapfrog Group Minimal Hospital and Surgeon Volume Thresholds for Esophagectomy Favor Specific Patient Demographics? Ann Surg 2021;274:e220-9. [Crossref] [PubMed]
- Patel DC, Jeffrey Yang CF, He H, et al. Influence of facility volume on long-term survival of patients undergoing esophagectomy for esophageal cancer. J Thorac Cardiovasc Surg 2022;163:1536-1546.e3. [Crossref] [PubMed]
- Markar SR, Lagergren J. Surgical and Surgeon-Related Factors Related to Long-Term Survival in Esophageal Cancer: A Review. Ann Surg Oncol 2020;27:718-23. [Crossref] [PubMed]
- van Workum F, Verstegen MHP, Klarenbeek BR, et al. Intrathoracic vs Cervical Anastomosis After Totally or Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer: A Randomized Clinical Trial. JAMA Surg 2021;156:601-10. [Crossref] [PubMed]
- Nguyen NT, Rudersdorf PD, Smith BR, et al. Management of gastrointestinal leaks after minimally invasive esophagectomy: conventional treatments vs. endoscopic stenting. J Gastrointest Surg 2011;15:1952-60. [Crossref] [PubMed]
- Athanasiou A, Hennessy M, Spartalis E, et al. Conduit necrosis following esophagectomy: An up-to-date literature review. World J Gastrointest Surg 2019;11:155-68. [Crossref] [PubMed]
- Helminen O, Kytö V, Kauppila JH, et al. Population-based study of anastomotic stricture rates after minimally invasive and open oesophagectomy for cancer. BJS Open 2019;3:634-40. [Crossref] [PubMed]
- Bhattacharyya N, Batirel H, Swanson SJ. Improved outcomes with early vocal fold medialization for vocal fold paralysis after thoracic surgery. Auris Nasus Larynx 2003;30:71-5. [Crossref] [PubMed]
Cite this article as: Elliott IA, Berry MF. A clinical practice review of the surgical approach for distal esophageal tumors. AME Med J 2023;8:5.

