Autologous blood patch pleurodesis for persistent air leak in the setting of chronic non-invasive ventilation: case report
Highlight box
Key findings
• Autologous blood patch pleurodesis may need to be attempted multiple times in ventilated patients with persistent air leaks and can have an immediate effect.
• The success of autologous blood patch pleurodesis may also be patient position dependent.
What is known and what is new?
• Autologous blood patch pleurodesis is a safe treatment option for persistent air leaks.
• Persistent air leaks in patients on positive pressure ventilation are much more difficult to treat and resolve.
What is the implication, and what should change now?
• Autologous blood patch pleurodesis’ effectiveness may be dependent on patient positioning; therefore, if a patient has an apical chest tube, an upright position may allow more even distribution of blood in the chest cavity.
Introduction
Persistent air leaks (PAL) are a known complication of spontaneous pneumothorax secondary to underlying lung disease, pulmonary infections, mechanical ventilation, chest trauma and pulmonary resections (1,2). Apart from surgical management, non-surgical treatment modalities described in the literature include autologous blood patch pleurodesis (ABPP), chemical pleurodesis, and endoscopic therapies (2-4). The use of ABPP has been previously described in literature for non-surgical etiologies of PAL, including secondary spontaneous pneumothorax from interstitial lung disease (5). ABPP has been demonstrated to be an effective and safe method in the treatment of PALs in secondary spontaneous pneumothorax (6-8), and the effectiveness and safety of ABPP may be similar to chemical pleurodesis (5). Several case studies have described the successful use of ABPP in coronavirus disease 2019 (COVID-19) patients with refractory pneumothorax who were unable to undergo surgical management (9,10).
To date, no literature has described the use of ABPP in patients requiring chronic non-invasive ventilation. We describe a patient who was dependent on non-invasive ventilation and subsequently developed a PAL, which resolved after ABPP was administered with the patient being in an upright position. We present the following case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-77/rc).
Case presentation
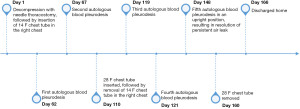
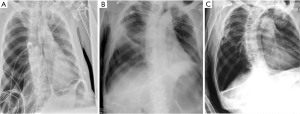
Our case is a 61-year-old male lifelong non-smoker with a history of amyotrophic lateral sclerosis (ALS), initially diagnosed in 2017. Due to neuromuscular weakness, he has been dependent on bilevel positive airway pressure ventilation since 2018. He had no history of prior lung disease, abnormal chest radiographs, and no history of pertinent occupational or environmental exposures. He was previously offered tracheostomy but declined it. Our case presented to our tertiary centre in 2021 with acute dyspnea, increased work of breathing and hypoxemia. His initial chest radiograph found that he developed his first secondary spontaneous pneumothorax and was felt to be in of tension due to increased oxygen requirements and hemodynamic instability (Figure 1A). The etiology behind his pneumothorax was not certain but it may have been due to barotrauma from bilevel positive airway pressure ventilation. He underwent needle decompression followed by insertion of a 14 F apical chest tube. While there was initially partial re-expansion of the right lung, it became evident that he developed a PAL as he had continuous air bubbling into the chest drainage for more than 5 days post-chest tube insertion.
Hospital course
The patient had a grade 4 PAL (2,11), or continuous bubbling present in the air leak chamber during both inspiration and expiration, that did not resolve for several months. Contributing factors included his dependence on bilevel positive airway pressure and mechanical exsufflation-insufflation for mucous clearance. He was not a surgical candidate due to his dependence on chronic non-invasive ventilation, immobility, and poor functional status. Due to his neuromuscular weakness, our patient has been bedbound for 3 years preceding his hospital admission and could not be weaned from non-invasive mechanical ventilation. The patient did not want to be intubated for endoscopic therapies due to risk of respiratory failure from sedation. He also declined chemical pleurodesis due to concerns of pain and risks related to sedation. The patient initially requested to continue with current catheter thoracostomy to avoid additional potential risks from other procedures, including ABPP.
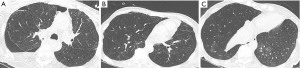
His computed tomography (CT) chest on day 12 found a small residual pneumothorax despite appropriately positioned right pleural drain, but there was no evidence of emphysema, bullae, bronchopleural fistulas, or interstitial lung disease. He required at least −20 cmH2O to maintain lung expansion. Given the limited treatment options available and his non-resolving PAL, on day 62 the patient agreed to trial of ABPP since sedation is not required for this treatment. For the ABPP protocol, 100 mL of venous blood was drawn from the peripherally inserted central catheter. The blood was immediately injected into the 14 F chest tube, followed by 30 mL saline flush. The chest tube, located in the 5th intercostal space over the right anterior axillary line, was elevated above the patient’s chest to avoid blood reflux into the tubing and plugging. It was also connected to gravity drainage to allow any air build up to escape if needed. After one hour, a chest radiograph was performed to reassess the pneumothorax.
The initial two ABPP procedures resulted in immediate resolution of the air leak (day 62 and 67). After each procedure, he developed hemoptysis within 30 min of injection of peripheral blood. While the presence of air leak through the drainage system ceased temporarily after instillation of blood, the leak resumed in less than 24 h. Since the leak was so large that there was incomplete lung expansion, his 14 F apical chest tube was exchanged on day 110 with a larger 28 F apical chest tube, which was effective to enable full lung expansion. The patient initially declined further therapies due to their associated risks, but he later agreed to repeat ABPP. Two more procedures were done with the larger tube (day 119 and 121), but once again, the leak resumed within 24 hours (Figure 1B).
In the first four procedures, our patient was in a supine position angled at 30 degrees. On day 148, the patient agreed to a fifth procedure with him supported in an upright position to allow more even distribution of blood in the hemithorax, given that the tip of the chest tube was at the apex. Once again, shortly after injecting the blood into his apical chest tube, he had small volume hemoptysis, indicating that the blood had reached the parenchymal tear. Similar to his previous trials of ABPP, he tolerated the procedure well with small volume hemoptysis as the only complication. The air leak and pneumothorax subsequently resolved on the same day of the procedure. To ensure long-term resolution of his PAL, his chest tube remained on continuous suction until after his repeat imaging study. The radiograph five days after the last ABPP found no recurrent pneumothorax. On day 160, his chest tube was removed after seven days of intermittent suctioning, as per his family’s request. His radiograph performed a day after his chest tube removal found no recurrent pneumothorax (Figure 1C) and he was later discharged from the hospital on day 166. Although he underwent multiple ABPP procedures, his hemoglobin levels were stable (between 80 to 100) and he did not require a blood transfusion during his hospital admission. He has not had recurrence of his pneumothorax with 1 year of follow-up based on our clinical assessment and repeat chest radiograph. Figure 2 includes CT chest contrast axial cuts prior to ABPP and Figure 3 outlines the timelines of events.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s next-of-kin for publication of this case report and any accompanying images. All means were taken to protect the identity of this patient and all patient identifiers were removed from the report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Air leaks result from the presence of an alveolar pleural fistula, which is where there is communication between the alveoli of the pulmonary parenchyma distal to a segmental bronchus with the pleural space (1). An air leak is considered to be persistent when it lasts beyond 5 to 7 days (2). Many guidelines recommend conservative watchful waiting with chest tube drainage followed by surgical repair as the gold standard for treatment for PAL (2). For patients who are non-surgical candidates, chemical pleurodesis, ABPP, or endobronchial therapies such as one-way bronchial valves are potential treatment options (2). To our knowledge, no research studies or case reports have described the use of ABPP in patients requiring chronic non-invasive ventilation who developed PAL.
Multiple case studies as well as two randomized controlled trials have reported success using ABPP (2,12-14). Using 100 mL of blood may be more effective than 50 mL (12). The blood may immediately patch the alveolar pleural fistula, and also induce inflammation and pleurodesis (15). The procedure is overall safe with only a few, rare complications, including chest tube obstruction, pleuritis, and empyema (2). In order to reduce the risk of tension pneumothorax caused by chest tube obstruction, peripheral venous blood should be rapidly administered through large bore pleural catheters, followed by a sterile saline flush (16-18). For our patient, we initially administered peripheral venous blood via a 14 F chest tube but it was later exchanged with a 28 F chest tube, since large bore pleural catheters are preferable for ABPP. While it has been suggested by nuclear medicine studies that deposition of injected fluid into the chest distributes regardless of positioning, our case highlights that it may impact efficacy, especially when using blood (19). However, since our observation cannot prove causality or generalizability, further studies are required to determine whether patient positioning is correlated with improved ABPP outcomes.
The management of PAL in our case study was particularly challenging as he was dependent on non-invasive mechanical ventilation. The positive pressure delivered by mechanical ventilation likely impairs the blood patch’s ability to “seal off” the leak, as the pressure will stress the leak towards the pleural space on inspiration and away from the pleural space on exhalation (20). This likely impaired the outcomes of the first four ABPP trials in our case study. Currently, there is limited data on the management of PAL in mechanically ventilated patients who fail to respond to conservative management. In critically ill patients requiring mechanical ventilation, ABPP or bronchoscopic therapies, including endobronchial valves, are available treatment options (20). Further research is required to determine the efficacy of these treatments in patients requiring chronic non-invasive mechanical ventilation.
Conclusions
To conclude, our case study suggests that ABPP can be used to treat PAL in patients requiring chronic non-invasive ventilation. ABPP is also likely an option for patients requiring invasive mechanical ventilation. Pleural blood patch pleurodesis may be particularly useful in fragile patients with PAL who are not surgical candidates, although multiple attempts may be required. Our case also highlights the importance of patient positioning in ABPP. Positioning the patient in an upright position, or a position that would permit the blood to distribute more evenly through the chest cavity may be more effective. This theory needs to be assessed further in prospective studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-77/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-77/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-77/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clark JM, Cooke DT, Brown LM. Management of Complications After Lung Resection: Prolonged Air Leak and Bronchopleural Fistula. Thorac Surg Clin 2020;30:347-58. [Crossref] [PubMed]
- Dugan KC, Laxmanan B, Murgu S, et al. Management of Persistent Air Leaks. Chest 2017;152:417-23. [Crossref] [PubMed]
- Travaline JM, McKenna RJ Jr, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest 2009;136:355-60. [Crossref] [PubMed]
- Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7; discussion 897-8. [Crossref] [PubMed]
- Aihara K, Handa T, Nagai S, et al. Efficacy of blood-patch pleurodesis for secondary spontaneous pneumothorax in interstitial lung disease. Intern Med 2011;50:1157-62. [Crossref] [PubMed]
- Akar E, Haberal MA, Şengören Dikiş Ö. The effectiveness of blood amount used in pleurodesis to prevent prolonged air leakage. Turk Gogus Kalp Damar Cerrahisi Derg 2020;28:175-80. [Crossref] [PubMed]
- Shaw JA, Wilken E, Allwood BW, et al. Autologous Blood Patch Pleurodesis for the Management of a Persistent Air Leak after Secondary Spontaneous Pneumothorax. Respiration 2022;101:417-21. [Crossref] [PubMed]
- Ibrahim IM, Elaziz MEA, El-Hag-Aly MA. Early Autologous Blood-Patch Pleurodesis versus Conservative Management for Treatment of Secondary Spontaneous Pneumothorax. Thorac Cardiovasc Surg 2019;67:222-6. [Crossref] [PubMed]
- Mitsuyama Y, Tanaka S, Ike A, et al. Refractory pneumothorax secondary to COVID-19 treated by autologous blood patch pleurodesis. QJM 2022;114:803-4. [Crossref] [PubMed]
- Fantin A, Castaldo N, Vailati P, et al. Full medical treatment of COVID-19 associated large pneumothorax – A case report. Monaldi Arch Chest Dis 2021;92: [Crossref] [PubMed]
- Cerfolio RJ, Tummala RP, Holman WL, et al. A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg 1998;66:1726-31. [Crossref] [PubMed]
- Andreetti C, Venuta F, Anile M, et al. Pleurodesis with an autologous blood patch to prevent persistent air leaks after lobectomy. J Thorac Cardiovasc Surg 2007;133:759-62. [Crossref] [PubMed]
- Shackcloth MJ, Poullis M, Jackson M, et al. Intrapleural instillation of autologous blood in the treatment of prolonged air leak after lobectomy: a prospective randomized controlled trial. Ann Thorac Surg 2006;82:1052-6. [Crossref] [PubMed]
- Lang-Lazdunski L, Coonar AS. A prospective study of autologous ‘blood patch’ pleurodesis for persistent air leak after pulmonary resection. Eur J Cardiothorac Surg 2004;26:897-900. [Crossref] [PubMed]
- Kurman JS. Persistent air leak management in critically ill patients. J Thorac Dis 2021;13:5223-31. [Crossref] [PubMed]
- Williams P, Laing R. Tension pneumothorax complicating autologous “blood patch” pleurodesis. Thorax 2005;60:1066-7. [Crossref] [PubMed]
- Botwin AL, Niedzwiecki GA, Botwin KP. Persistent air leak treated by autologous blood patch pleurodesis: the role of CT-guided small-bore chest tube insertion. Clin Imaging 2020;60:75-8. [Crossref] [PubMed]
- Cobanoglu U, Melek M, Edirne Y. Autologous blood pleurodesis: A good choice in patients with persistent air leak. Ann Thorac Med 2009;4:182-6. [Crossref] [PubMed]
- Baas P, Neijens VH, Olmos RA, et al. Distribution of 99mTc colloid in the thoracic cavity of patients with malignant pleural effusions. Lung Cancer 1997;17:239-47. [Crossref] [PubMed]
- Durrance RJ, D’Souza KG, Obata R, et al. Endobronchial blood-patch: A novel technique for a persistent pleural air leak. Respir Med Case Rep 2022;38:101670. [Crossref] [PubMed]
Cite this article as: Sekowski V, Li P. Autologous blood patch pleurodesis for persistent air leak in the setting of chronic non-invasive ventilation: case report. AME Med J 2023;8:9.