Mendelian randomization found no causal relationship between omega-6 fatty acids and attention deficit hyperactivity disorder (ADHD)
Highlight box
Key findings
• Using two-sample Mendelian randomization (MR) method, this study finds no potential treatment impact of omega-6 on Attention deficit hyperactivity disorder (ADHD).
What is known and what is new?
• Studies have indicated that increasing one’s intake of foods rich in omega-3, omega-6, and omega-9 fatty acids improves one’s mental and physical well-being.
• The results were confirmed by using data from the ADHD GWAS, which comprised 20,138 cases and 35,191 controls for comparison. ADHD GWAS data, which included 20,138 cases and 35,191 controls, was used to corroborate the findings.
• By removing SNPs with incompatible alleles and palindromic SNPs with intermediate allele frequencies, the SNPs in the exposure and outcome datasets were harmonized (minor allele frequency threshold =0.01), resulting in a final set of 8 SNPs used for MR analysis (accounting for 4.6% of omega-6 fatty acids variance, R2=0.046). Thus, there is no evidence from our study that omega-6 fatty acids contribute to treatment of ADHD.
What is the implication, and what should change now?
• Due to a lack of supporting data, the use of omega-6 fatty acids in the treatment of ADHD is not currently recommended.
Introduction
Long-chain polyunsaturated fatty acids (LCPUFA), such as omega-3 and omega-6, are important building blocks of cell membranes. Diet, which in Western nations is thought to have an omega-3:omega-6 ratio of 15–20:1, is the main source of these LCPUFA in humans (1). While over 5% of children and adolescents are diagnosed with attention deficit hyperactivity disorder (ADHD), making it one of the most prevalent mental health issues in pediatrics and adolescence. It is characterized by persistent attention deficit, hyperactivity, and impulsivity (2). ADHD is more prevalent in boys than in girls. Its specific cause is unknown. However, new research indicates that various gene and non-inherited factors play a significant role in ADHD (3). Compared to children without signs of ADHD, children with symptoms had greater omega-6 ratios, which may be related to dietary habits, changed gut flora, or abnormal LCPUFA metabolism (4).
Children with ADHD have underlying psychiatric problems such as bipolar disorder, schizophrenia, impulsive behaviour, and functional impairment (5). Although ADHD affects children aged 6–17 years. Most children with ADHD have not been fully cured, with estimates ranging from 60% to 80%. These symptoms will last till adulthood; many recent studies highlighted that adults with ADHD are at high risk of psychiatric disorders, including anxiety, depression, and suicidality (6). Human infants require omega-6 and omega-3 polyunsaturated fatty acids (PUFA) for neurodevelopment as well as to maintain neural integrity and function (7).
Although the pathogenesis of ADHD is not fully understood, current research suggests that catecholamines, particularly noradrenaline and dopamine, play an important role in treatment. Common pharmaceutical treatments for ADHD include methylphenidate and atomoxetine, which are essential in reducing symptoms (8). Recent randomized controlled trials (RCTs) suggest that omega-6 fatty acid supplementation may play a crucial role in improving cognitive function and behaviour in patients with neurological disorders (9,10). The omega-6 fatty acid gamma-linolenic acid (GLE) plays a crucial role in the formation of the brain-abundant arachidonic acid (ARA) (11,12). Along with omega-6 fatty acids, brain lipids with the cell membrane serve as a signalling medium, which is considered to play a crucial role in the prevention of anxiety disorders (13). However, some meta-analyses indicate that children and adults with ADHD have elevated ratios of blood omega-6 to omega-3, indicating a disruption in fatty acid metabolism (14). Although research on drug treatment is still in its early stages, some parents will select behaviour therapy for their children with ADHD; studies have shown that omega-3/6 supplementation reduces behavioural and learning difficulties in children with ADHD (15-19). Mendelian randomization (MR) is a method used to assess the association between exposure and result; in this study, genetic differences, or single nucleotide polymorphisms (SNPs), serve as instrument variables (IVs). The purpose of this study is to design a two-sample MR to explore the relationship between omega-6 fatty acids and ADHD. This manuscript is written following the STROBE-MR reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-74/rc).
Methods
Study design
MR analyses is a method commonly used in epidemiology to estimates the causal relationship between exposure and disease based on natural experiments assigned to high and low levels of exposure depending on individual genetic variation. The MR base platform was used to download data on SNPs related to omega-6 fatty acids, which were then analysed with R software (version 4.0.5). The MR base platform (http://www.mrbase.org) is a computer program and online medium for MR database and analysis developed by the integrative epidemiology unit of the University of Bristol’s Medical Research Council (MRC) (20). The exposed cohort was drawn from the genome-wide association study (GWAS) cohort reported by Kettunen et al. in 2016 (21). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Sample population, size, and procedure
The sample population was European, and the sample size was 13,506. A clumping procedure was performed to retain the SNPs that were related to omega-6 fatty acids at a genome-wide significance level of P<5×10−8. SNPs were in linkage disequilibrium with the index SNPs (r2 threshold ≤0.001) or within a 10,000 kb distance.
Furthermore, the outcome cohort was a GWAS meta-analysis of 12 cohorts that identified the first genome-wide significant ADHD loci and included 20,138 cases and 35,191 controls (22). Most people in the sample were European (approximately 95%). Patients with ADHD were found in the national Psychiatric Central Research Register and diagnosed by psychiatrists in a psychiatric hospital using the ICD10 code.
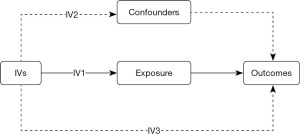
MR is only a reliable method of making causal inferences if three conditions are met. The SNPs utilized in the IV relate to the exposure; there are no known or unknown confounders of the result, and the SNPs affect the outcome only through the pathway of interest (Figure 1).
Assumption of two-sample MR
MR necessitates that genetic instruments are related with a modifiable exposure of interest (assumption 1), and any relationship between the instruments and outcome is mediated by the exposure (assumption 2). The instrumental variable only affects the outcome through its effect on risk factors (assumption 3) (23). SNPs that violate these assumptions are called invalid instrumental variable and considering it in MR analyses may lead to a deviation in the results. In the absence of reliable information on the ratio and direction of functional pathways and various genetic variations, additional MR methods, including the weighted median of MR-Egger, were used to check the consistency of the estimated direction of the effect (24).
Inverse variance weighted (IVW) meta-analysis was used to combine MR estimates for each probable direction of influence, which is essentially a weighted regression of SNP-outcome effects on SNP-exposure effects with a zero intercept (25). Outcomes may be biased if instrument SNPs exhibit horizontal pleiotropy or they influence outcomes through causal pathways other than exposure (26). We compared IVW findings with that of other MR methods, whose estimates are known to be less affected by horizontal pleiotropy. Two orthogonal methods were used (weighted median estimator, which is consistent even when up to 50% of the information comes from invalid IVs (24). and MR-Egger, which provides a valid test of directional (unbalanced) pleiotropy, and an accurate test of the causal null hypothesis) to perform additional sensitivity analyses since the IVW method requires that all IVs meet all the MR assumptions.
Statistical analysis
We evaluated the weak instrument bias by F statistics which was calculated using the following formula, where k is the number of SNPs and n is the sample size, R2 refers to the variance explained by SNPs in omega-6 fatty acids (25).
Further tests for horizontal pleiotropy were performed using the MR-Egger intercept test of deviation from the null to assess the robustness and significance of the results. Also, leave-1-SNP-out analyses were performed to check whether SNPs influence the overall estimates.
Results
The SNPs were harmonized (minor allele frequency threshold =0.01) between the exposure and outcome data sets by removing SNPs with incompatible alleles as well as palindromic SNPs with intermediate allele frequencies, resulting in a final set of 8 SNPs used for MR analysis (accounting for 4.6% of omega-6 fatty acids variance, R2=0.046).
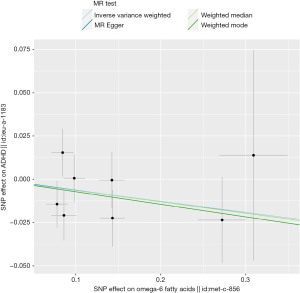
There is no significant relationship between omega-6 fatty acids and ADHD. The odds ratio (OR) of the 8-SNP IVs based the IVW analysis was 0.94 per standard deviation (SD) of omega-6 fatty acids, 95% CI, 0.86–1.02, P=0.17 (Figure 2). Other analyses revealed comparable findings (MR Egger: P=0.59, 0.94 per SD of omega-6 fatty acids, 95% CI, 0.75–1.18; weighted median: P=0.27, 0.94 per SD of omega-6 fatty acids, 95% CI, 0.83–1.05; weighted model: P=0.39, 0.93 per SD of omega-6 fatty acids, 95% CI, 0.79–1.09) (Table 1).
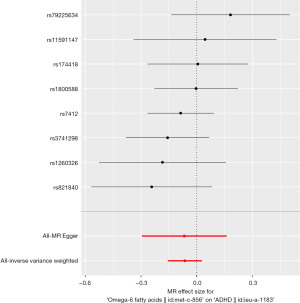
Table 1
| Method | OR | 95% CI | P value |
|---|---|---|---|
| MR Egger | 0.94 | 0.75–1.18 | 0.59 |
| Weighted median | 0.94 | 0.83–1.05 | 0.27 |
| Inverse variance weighted | 0.94 | 0.86–1.02 | 0.17 |
| Weighted mode | 0.93 | 0.79–1.09 | 0.39 |
ADHD, attention deficit hyperactivity disorder; MR, Mendelian randomization; OR, odds ratio; CI, confidence interval.
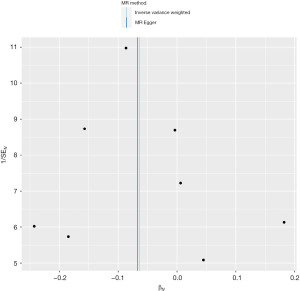
The F statistics were 81, greater than 10, demonstrating the insignificant, weak instrument bias. Based on Cochran’s Q, there was no significant heterogeneity in Wald ratios for individual SNPs in the IV (P=0.602 for IVW; P=0.485 for MR-Egger). The MR-Egger intercept test (Intercept =0.00038, P=0.979) revealed no strong directional horizontal pleiotropy (Figure 3).
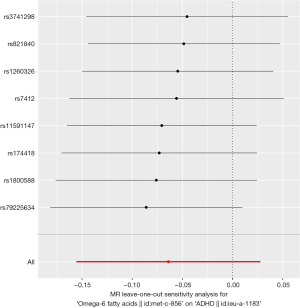
Furthermore, funnel plot and leave-one-out sensitivity analysis using the IVW method revealed no single SNPs influenced the estimate results (Figures 4,5).
Discussion
This study reveals no potential treatment impact of omega-6 on ADHD incidence. The negative outcome of this study contradicts the findings of several prior investigations. ADHD is one of the most common neurodevelopment disorders, affecting between 5% and 12% of children (27). Although the fundamental cause of ADHD is unknown and numerous factors contribute to its progression, genetic and environmental factors may increase the chance of developing the condition. Recently, the connection between nutritional supplements and ADHD has been analyzed (28). Omega-6 fatty acid GLE play an essential role in the production of ARA which is present in brain in plenty amount Omega-6 fatty acid supplements may play a key role to improve cognitive function and behaviour in brain disorder.
Growing evidence and reports have shown that omega-3 and omega-6 PUFAs might be a potential treatment option for children with ADHD compared to typical medication, including methylphenidate hydrochloride and atomoxetine. Numerous cohort studies and meta-analyses published in the last decades have estimated pharmacological, non-pharmacological, and combined treatment efficacy and safety for managing ADHD (29-38). Increasing symptoms of ADHD at age 7 were associated with an increase in omega-6 to omega-3 LCPUFA in cord plasma. This shows that supplementing a mother’s diet with omega-6 fatty acids can lessen her child’s risk of developing ADHD (1).
The evidence supporting the benefits of DHA and EPA on fetal and infant brain development is increasing, and pregnant women are increasingly being encouraged to consume them (39). A comprehensive review of RCTs was designed based on these findings. Muhammad Abdullah et al. examined the effects of omega-3&6 based on the mean difference in Conners’ rating scale and seven trials. They conclude that there is insufficient evidence to determine if omega-3&6 supplementation is useful in lowering the symptoms of ADHD in children and adolescents (40). Renata Ramalho et al. analyzed nine studies, including five RCTs and four cohort studies, and identified three studies with negative results (41). In an intriguing study comparing the efficacy and safety of omega-3/6 fatty acids, methylphenidate, and combination treatment in children with ADHD, the effects of monotherapy methylphenidate were comparable to the effects of a combination of omega-3/6 fatty acids. Still, the methylphenidate (MPH) + omega combination demonstrated some unexpected benefits over methylphenidate (10). However, there is still no unified recommendation guideline or strong evidence for daily supplementary dose (42).
Limitations of this study
Likewise, our research has certain limitations. First, the MR analysis is predicated on three tight assumptions. However, we minimize their effect by employing several models and sensitivity analysis, we had limited power to detect causal effects for some traits. Second, MR methods depend on the source GWAS sample sizes and trait heritability. These factors had a particular impact on the GWAS summary data sets for attention, and intra-individual response time variability, as all GWAS were based on modestly sized samples. Many cognitive traits associated with ADHD, such as reward processing, visuospatial working memory, and sustained attention performance, still lack the publicly available GWAS data needed for MR. Thirdly, despite applying several methods to test for pleiotropy and finding limited evidence for any directional pleiotropy, we cannot entirely rule out possible bias due to residual horizontal pleiotropy. Fourth, only Europeans are allowed to participate; the outcome is not stable following a sensitivity analysis in which SNPs are eliminated one by one.
Conclusions
Even though we did not find evidence that omega-6 had a therapeutic effect on ADHD, our results may provide light on why those with the disorder also tend to experience several co-occurring issues as they grow older age. They may hold promise as a fresh starting point for research into developing preventative medicines for ADHD and the potentially damaging effects of the disorder.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE-MR reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-74/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-74/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-74/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- López-Vicente M, Ribas Fitó N, Vilor-Tejedor N, et al. Prenatal Omega-6:Omega-3 Ratio and Attention Deficit and Hyperactivity Disorder Symptoms. J Pediatr 2019;209:204-211.e4. [Crossref] [PubMed]
- Drechsler R, Brem S, Brandeis D, et al. ADHD: Current Concepts and Treatments in Children and Adolescents. Neuropediatrics 2020;51:315-35. [Crossref] [PubMed]
- Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet 2016;387:1240-50. [Crossref] [PubMed]
- Stevens LJ, Zentall SS, Deck JL, et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr 1995;62:761-8. [Crossref] [PubMed]
- Antshel KM, Barkley R. Attention deficit hyperactivity disorder. Handb Clin Neurol 2020;174:37-45. [Crossref] [PubMed]
- Hackett A, Joseph R, Robinson K, et al. Adult attention deficit/hyperactivity disorder in the ambulatory care setting. JAAPA 2020;33:12-6. [Crossref] [PubMed]
- Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 2012;2012:CD007986. [Crossref] [PubMed]
- Padilha SCOS, Virtuoso S, Tonin FS, et al. Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis. Eur Child Adolesc Psychiatry 2018;27:1335-45. [Crossref] [PubMed]
- Döpfner M, Dose C, Breuer D, et al. Efficacy of Omega-3/Omega-6 Fatty Acids in Preschool Children at Risk of ADHD: A Randomized Placebo-Controlled Trial. J Atten Disord 2021;25:1096-106. [Crossref] [PubMed]
- Barragán E, Breuer D, Döpfner M. Efficacy and Safety of Omega-3/6 Fatty Acids, Methylphenidate, and a Combined Treatment in Children With ADHD. J Atten Disord 2017;21:433-41. [Crossref] [PubMed]
- Dobryniewski J, Szajda SD, Waszkiewicz N, et al. Biology of essential fatty acids (EFA). Przegl Lek 2007;64:91-9.
- Alashmali SM, Hopperton KE, Bazinet RP. Lowering dietary n-6 polyunsaturated fatty acids: interaction with brain arachidonic and docosahexaenoic acids. Curr Opin Lipidol 2016;27:54-66. [Crossref] [PubMed]
- Müller CP, Reichel M, Mühle C, et al. Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta 2015;1851:1052-65. [Crossref] [PubMed]
- LaChance L, McKenzie K, Taylor VH, et al. Omega-6 to Omega-3 Fatty Acid Ratio in Patients with ADHD: A Meta-Analysis. J Can Acad Child Adolesc Psychiatry 2016;25:87-96.
- Perera H, Jeewandara KC, Seneviratne S, et al. Combined ω3 and ω6 supplementation in children with attention-deficit hyperactivity disorder (ADHD) refractory to methylphenidate treatment: a double-blind, placebo-controlled study. J Child Neurol 2012;27:747-53. [Crossref] [PubMed]
- Innis SM. Fatty acids and early human development. Early Hum Dev 2007;83:761-6. [Crossref] [PubMed]
- Antalis CJ, Stevens LJ, Campbell M, et al. Omega-3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids 2006;75:299-308. [Crossref] [PubMed]
- Germano M, Meleleo D, Montorfano G, et al. Plasma, red blood cells phospholipids and clinical evaluation after long chain omega-3 supplementation in children with attention deficit hyperactivity disorder (ADHD). Nutr Neurosci 2007;10:1-9. [Crossref] [PubMed]
- McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 2006;75:329-49. [Crossref] [PubMed]
- Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. [Crossref] [PubMed]
- Kettunen J, Demirkan A, Würtz P, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun 2016;7:11122. [Crossref] [PubMed]
- Demontis D, Walters RK, Martin J, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 2019;51:63-75. [Crossref] [PubMed]
- Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res 2007;16:309-30. [Crossref] [PubMed]
- Bowden J, Davey Smith G, Haycock PC, et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol 2016;40:304-14. [Crossref] [PubMed]
- Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37:658-65. [Crossref] [PubMed]
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512-25. [Crossref] [PubMed]
- Mohammadzadeh S, Baghi N, Yousefi F, et al. Effect of omega-3 plus methylphenidate as an alternative therapy to reduce attention deficit-hyperactivity disorder in children. Korean J Pediatr 2019;62:360-6. [Crossref] [PubMed]
- Del-Ponte B, Anselmi L, Assunção MCF, et al. Sugar consumption and attention-deficit/hyperactivity disorder (ADHD): A birth cohort study. J Affect Disord 2019;243:290-6. [Crossref] [PubMed]
- Caye A, Swanson JM, Coghill D, et al. Treatment strategies for ADHD: an evidence-based guide to select optimal treatment. Mol Psychiatry 2019;24:390-408. [Crossref] [PubMed]
- Catalá-López F, Hutton B, Núñez-Beltrán A, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS One 2017;12:e0180355. [Crossref] [PubMed]
- Storebø OJ, Krogh HB, Ramstad E, et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 2015;351:h5203. [Crossref] [PubMed]
- Cortese S, Ferrin M, Brandeis D, et al. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry 2015;54:164-74. [Crossref] [PubMed]
- Daley D, van der Oord S, Ferrin M, et al. Behavioral interventions in attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry 2014;53:835-47, 847.e1-5.
- Riera M, Castells X, Tobias A, et al. Discontinuation of pharmacological treatment of children and adolescents with attention deficit hyperactivity disorder: meta-analysis of 63 studies enrolling 11,788 patients. Psychopharmacology (Berl) 2017;234:2657-71. [Crossref] [PubMed]
- Zwi M, Jones H, Thorgaard C, et al. Parent training interventions for Attention Deficit Hyperactivity Disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst Rev 2011;2011:CD003018. [Crossref] [PubMed]
- Storebø OJ, Skoog M, Damm D, et al. Social skills training for Attention Deficit Hyperactivity Disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst Rev 2011;CD008223. [Crossref] [PubMed]
- Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: psychostimulants, alpha-2 agonists, and atomoxetine. Can J Psychiatry 2015;60:42-51. [Crossref] [PubMed]
- Liu Q, Zhang H, Fang Q, et al. Comparative efficacy and safety of methylphenidate and atomoxetine for attention-deficit hyperactivity disorder in children and adolescents: Meta-analysis based on head-to-head trials. J Clin Exp Neuropsychol 2017;39:854-65. [Crossref] [PubMed]
- Cagigal C, Silva T, Jesus M, et al. Does Diet Affect the Symptoms of ADHD? Curr Pharm Biotechnol 2019;20:130-6. [Crossref] [PubMed]
- Abdullah M, Jowett B, Whittaker PJ, et al. The effectiveness of omega-3 supplementation in reducing ADHD associated symptoms in children as measured by the Conners' rating scales: A systematic review of randomized controlled trials. J Psychiatr Res 2019;110:64-73. [Crossref] [PubMed]
- Ramalho R, Pereira AC, Vicente F, et al. Docosahexaenoic acid supplementation for children with attention deficit hyperactivity disorder: A comprehensive review of the evidence. Clin Nutr ESPEN 2018;25:1-7. [Crossref] [PubMed]
- Firth J, Teasdale SB, Allott K, et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019;18:308-24. [Crossref] [PubMed]
Cite this article as: Saeed S, Jiang L, Xu J, Wang G, Leng M, Wu J, Qian S, Jiang CM. Mendelian randomization found no causal relationship between omega-6 fatty acids and attention deficit hyperactivity disorder (ADHD). AME Med J 2023;8:3.