
Therapy interruptions and drug interactions during acute hospitalization in patients taking direct acting antivirals for chronic hepatitis C virus infection: a retrospective cross-sectional study
Highlight box
Key findings
• Drug interactions with DAA therapy are common;
• DAA therapy is a robust treatment for chronic HCV with short treatment interruptions and minor drug interactions unlikely to significantly impair treatment efficacy.
What is known and what is new?
• Patients admitted to hospital while taking DAAs are at high risk of drug interactions;
• Majority of drug interactions are not identified or properly addressed by clinicians;
• Some of the well described pharmacokinetic drug interactions involving DAA therapy may not be clinically relevant.
What is the implication, and what should change now?
• All patients admitted to hospital while taking DAA therapy should be referred for assessment by an expert in HCV pharmacotherapy due to the frequency of drug interactions with other common medications.
Introduction
Chronic hepatitis C virus (HCV) infection is estimated to affect roughly 58 million people (1). It is believed that only 20% of individuals infected with HCV are aware of their diagnosis, and only 60% of those have had the opportunity to receive direct-acting antiviral (DAA) therapy (1). With more than 50 million untreated cases of HCV worldwide, rapid and safe access to effective DAAs is needed to achieve the World Health Organization (WHO)’s 2030 goal of reducing HCV elimination target.
Since 2014, highly effective DAAs have been available for the treatment of HCV. Efficacy rates in clinical trials for the various available DAAs are in excess of 90% (2). Real-world effectiveness of DAA therapy ranges from 85–95% depending on the treatment setting, patient demographics, and HCV genotype (3). Until recently, DAA prescribing occurred primarily in specialized outpatient clinics under the supervision of gastroenterologists or infectious diseases specialists. Current Canadian HCV treatment guidelines have recommended the transition of HCV care from specialty clinics to primary care settings (4). As access and prescribing is expanded to a broader range of patients, we can expect to see an increase in the number of patients admitted to hospital while on DAA therapy (5,6).
The list price of a single pill of contemporary DAA regimens costs between $600–$1,000 (CAD) and the majority of treatment courses are for 12 weeks (7,8). Due to the significant financial burden of these therapies, many hospitals do not stock or supply DAA therapies to admitted patients (9). In most circumstances patients are expected to supply their own medications while in hospital. This may lead to treatment interruptions which in turn can threaten treatment success. It has been reported that patients who miss as little as 10% of their regimen are at an increased risk of treatment failure (10). Adherence to DAA therapy typically ranges between 60–90% depending on patients’ socioeconomic and comorbid mental health status and presence of substance use disorders (11-13). A significant gap exists regarding adherence rates in patients receiving DAA therapies outside of specialized clinics. To our knowledge, there is no literature describing adherence rates in patients admitted to hospital while taking DAA therapy.
In addition to the risks associated with treatment interruption, DAAs are also commonly implicated in drug interactions which are often present in hospitalized patients with multiple comorbid conditions (14). In the outpatient setting, between 30–60% of patients prescribed DAAs are at risk of clinically significant drug interactions (15-17). The prevalence of clinically significant drug interactions in patients admitted to hospital while taking DAA therapy has not been previously described. Given their novelty and rarity, it can be expected that most physicians and pharmacists outside of specialized practice areas have limited knowledge of HCV pharmacotherapy related interactions and mitigation strategies.
Our primary objective was to determine the frequency of DAA therapy interruptions in patients who are hospitalized while receiving HCV treatment. We also aimed to quantify and characterize drug interactions and their management strategies in patients who are admitted to the hospital while receiving HCV DAAs. We present the following article in accordance with the STROBE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-80/rc).
Methods
We designed a retrospective cross-sectional study including all patients hospitalized while receiving active DAA therapy during a 3.5-year period. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the UBC Clinical Research Ethics Board and the Vancouver Coastal Health Research Institute (No. H18-02567). Individual consent for this retrospective analysis was waived.
All adult patients admitted to Vancouver General Hospital (VGH) between January 1st, 2015 and June 30th, 2018 were screened for inclusion into our study. Eligibility criteria included all adults older than 18 years of age and receiving active DAA therapy as an inpatient. We generated a report through electronic medical record (EMR) that identified all patients ordered at least one of the following medications to be continued while patients are admitted: asunepravir, glecaprevir, grazoprevir, paritaprevir, simeprevir, voxilaprevir, daclatasvir, elbasvir, ledipasvir, ombitasvir, pibrentasvir, velpatasvir, dasabuvir, or sofosbuvir. Patients were excluded if DAA therapy was initiated in hospital. We also excluded patients receiving DAA therapy as part of clinical trials since these patients would be managed closely by dedicated clinical trials nurses and experts in HCV therapy and will not represent real world data. We then linked our database to the provincial virology laboratory, the British Columbia Centre for Disease Control database, to obtain HCV genotyping, and sustained virological response at week 24 (SVR24) data for each patient.
Baseline characteristics including age, sex, dates of admission and discharge, admitting diagnosis, DAA regimen, medication list, and laboratory values were extracted electronically. Physical charts and in-patient EMR were manually reviewed to determine baseline Child-Pugh, MELD, and MELD-Na scores, as well as identifying timelines for DAA orders written, processed, and administered. Therapy was considered to have been interrupted if more than 24 hours elapsed between the time of presentation to hospital and administration of their next DAA dose. Interruptions were classified as intentional if the medication was not continued on admission medication reconciliation, and unintentional if the physician ordered the DAA therapy on admission but administration was delayed for reasons such as difficulty procuring patient’s own supply. Drug interactions were manually assessed using the University of Liverpool HEP Drug Interactions online tool, and well as Lexi-Interact® (18,19). Interactions were recorded according to category and severity according to University of Liverpool HEP Drug Interactions. In the event of lack of information or incongruence between the interaction tools, an expert in HCV pharmacotherapy made the final determination.
Statistical analysis
Given the descriptive nature of this retrospective cross-sectional study, no sample size calculations were carried out. Period prevalence of DAA treatment interruptions and drug-drug interactions were determined. Numerical data are presented as means with standard deviations when normally distributed and nonparametric data as medians with the interquartile ranges (IQR). Categorical data are presented with their corresponding percentages.
Results
During the study period, 61 patients were prescribed DAA regimens while admitted to the hospital. Of those, six patients were excluded as their DAA regimen was initiated during the index hospitalization. We were unable to locate the physical chart for one patient and therefore they were excluded from the study. Three patients were admitted multiple times, as such, 54 unique patients contributed to a total of 57 hospitalizations (Figure 1). Baseline characteristics of patients on admission are summarized in Table 1. The majority of patients were men (41/54), the median age was 60 (IQR, 53–66), and the median MELD-Na score at admission was 13 (IQR, 10–20). Almost half the patients (25/54) had decompensated cirrhosis (Child Pugh Class B or C) and were hospitalized for a liver related indication and nearly 10% required admissions to critical care unit. The most common DAA combination was sofosbuvir/ledipasvir with or without ribavirin (30/54).
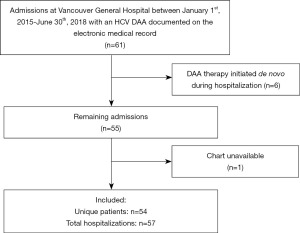
Table 1
| Variables | Value |
|---|---|
| Participants, n | 61 |
| Age (years), median [IQR] | 60 [53–66] |
| Sex (male), n (%) | 41 (75.9) |
| MELD-Na on admission, median | 13 |
| Child-Turcotte-Pugh class, n (%) | |
| A | 20 (37.0) |
| B | 14 (25.9) |
| C | 11 (20.4) |
| Unable to assess | 9 (16.7) |
| Liver related hospitalization, n (%) | 25 (46.3) |
| Direct admission to ICU, n (%) | 5 (9.3) |
| Duration of hospitalization (days), median [IQR] | 6 [3–17] |
| HCV genotype, n | |
| 1a | 15 |
| 1b | 4 |
| 2 | 2 |
| 3 | 12 |
| Genotyping not available | 19 |
| Treatment regimen, n (%) | |
| Sofosbuvir/ledipasvir | 28 (51.9) |
| Sofosbuvir/ledipasvir + ribavirin | 2 (3.7) |
| Sofosbuvir/velpatasvir | 7 (13.0) |
| Sofosbuvir/velpatasvir + ribavirin | 2 (3.7) |
| Elbasvir/grazoprevir | 3 (5.6) |
| Elbasvir/grazoprevir + sofosbuvir | 2 (3.7) |
| Elbasvir/grazoprevir + sofosbuvir + ribavirin | 1 (1.9) |
| Sofosbuvir + ribavirin | 8 (14.8) |
| Sofosbuvir + daclatasvir + ribavirin | 1 (1.9) |
IQR, interquartile range; MELD-Na, Model for End-Stage Liver Disease Na Score; ICU, intensive care unit; HCV, hepatitis C virus.
DAA therapy was continued without interruption in 68.4% (39/57) of hospitalizations. Unintentional interruptions occurred in 24.6% (14/57) of cases while there were a total of four intentional interruptions accounting for 7.0% of hospitalization episodes (Table 2). Of the intentional interruptions in two cases the DAA was held for pre-operative “nothing by mouth” (NPO) status, in one case the DAA was held for prolonged peri-operative NPO status, and in the fourth case the DAA was held due to an interaction between the DAA and phenytoin. Two patients (3.5%) had their DAA therapy held in excess of 5 days. The documented indications for DAA therapy cessation were patient refusal to take medications by mouth, and concurrent phenytoin use. In the case of the patient on phenytoin a clinical pharmacist recommended using an alternative antiepileptic agent rather than interrupting DAA treatment; however, this recommendation was not implemented. In the event of therapy interruption, the median time to DAA administration after admission was 1 day (IQR, 0–1 day), the longest interruption documented was 46 days (range, 0–46 days). The majority of interruptions were a result of logistical delays in obtaining the patient’s own supply of anti-viral medication; however, in the case of the 46-day interruption the patient refused oral medications.
Table 2
| Variables | Value |
|---|---|
| Unintentional interruption on admission, n (%) | 14 (24.6) |
| Intentional interruption on admission, n (%) | 4 (7.0) |
| No interruption on admission, n (%) | 39 (68.4) |
| Median delay in DAA administration (days) [IQR] | 1 [0–1] |
DAA, direct-acting antiviral; IQR, interquartile range.
Drug interactions involving DAA therapy occurred during 63% (36/57) of hospitalizations. In total, 61 drug interactions occurred with 20 medications (Table 3). The most common DAA associated with drug interactions were the NS5A inhibitors ledipasvir and velpatasvir which were involved in 85% of all interaction. The most common drug interactions involved the coadministration of NS5A inhibitors and acid suppressing medications like proton-pump inhibitors (PPI), H2 receptor antagonists, and antacids (61%). The second and third most common interacting medications, also involving the NS5A inhibitors, were tenofovir disoproxil fumarate (8%), and statins (7%) respectively. Drug interactions occurred as a result of medications initiated while in hospital in 62% of cases, while the remaining interactions were pre-existing on admission (Table 4). A total of 54% of interacting drugs were newly initiated in hospital and were not ordered as part of a pre-printed order set or bundles that are automatically ordered upon admission or following specific procedures. Two drug interactions were identified as combinations that should not be co-administered, these were interactions between sofosbuvir and amiodarone, and sofosbuvir/ledipasvir and phenytoin. The remaining drug interactions were identified as being potentially clinically significant. The most common effects of the drug interactions were noted to be decreases in DAA absorption (Table 4).
Table 3
| Interacting drug (n=61) | Number |
|---|---|
| Pantoprazole | 15 |
| Ranitidine | 9 |
| Esomeprazole | 8 |
| Tenofovir disoproxil fumarate | 5 |
| Amlodipine | 4 |
| Calcium carbonate | 4 |
| Atorvastatin | 3 |
| Levothyroxine | 2 |
| Midazolam | 2 |
| Amiodarone | 1 |
| Aluminum hydroxide | 1 |
| Loperamide | 1 |
| Nadolol | 1 |
| Oxycodone | 1 |
| Phenytoin | 1 |
| Rosuvastatin | 1 |
| Tacrolimus | 1 |
| Warfarin | 1 |
DAA, direct-acting antiviral.
Table 4
| Characteristics | Number |
|---|---|
| Presence of drug interaction, n (%) | |
| Yes | 36 (63.2) |
| No | 21 (36.8) |
| Total number of drug interactions | 61 |
| Interaction present on admission | 23 (35.4) |
| Method of ordering interacting drug, n (%) | |
| Admission medication reconciliation | 14 (23.0) |
| Manually ordered | 33 (54.1) |
| Initiated as part of an order set | 14 (23.0) |
| Severity of drug interaction, n | |
| Potentially clinically significant | 59 |
| Do not co-administer | 2 |
| Effect of drug interaction, n | |
| Decreased absorption | 37 |
| Decreased clearance | 4 |
| Increased clearance | 1 |
| Severe bradycardia | 1 |
| Effects of other drug (non-DAA) | 18 |
| Resolution of drug interaction, n | |
| Unresolved | 43 |
| Resolved by clinician | 13 |
| Unintentional resolution | 5 |
DAA, direct-acting antiviral.
Pharmacists and physicians identified and resolved 21.3% of drug interactions. The remaining 78.7% of interactions were not documented as having been assessed by any clinician. Of the two contraindicated interactions, one was identified, and one was not. In the case of the identified interaction, the DAA was intentionally held for 22 consecutive days until the offending drug was discontinued. There was no documentation to suggest that the interactions were identified by any clinician in the remaining cases; however, in 8.2% of cases the drug interaction was unintentionally resolved through alterations in medication regimen that were documented to have occurred for another reason.
Despite therapy interruptions and drug interactions, 53 out of 54 patients achieved SVR24. One patient passed away while in hospital and did not have virologic evidence of HCV clearance. This patient did not have any DAA therapy interruption on admission; however, their DAA regimen was discontinued 15 days after admission when the patient was transitioned to palliative care for end-stage metastatic lung cancer.
Discussion
Our study provides the first real-world data on the frequency of drug interactions and therapy interruptions in patients who are hospitalized while receiving active DAA regimens for the treatment of chronic hepatitis C. Our results show that approximately one-third of patients admitted to hospital experience HCV treatment interruptions with the majority of cases (14/18) representing an unintentional therapy cessation. Despite the frequency, the median duration of interruption was only 1 day (IQR, 0–1 day). Nearly two-thirds of patients were prescribed at least one new interacting medication during their hospital admission. Most drug interactions (78.7%) were not discovered nor addressed by clinicians. However, despite frequent treatment interruptions and prevalent drug interactions, all patients except one (98%) achieved SVR24.
Due to significant expense of DAA therapies, most hospitals do not stock or supply DAAs to inpatients outside of exceptional circumstances. As such, patients must typically supply their own DAA following admission. These logistical challenges in obtaining medications can lead to temporary interruption in therapy. In our study, the majority of treatment interruptions were for a short period of 24–48 hours typically leading to a single missed dose. Similar durations of therapy interruption have been previously reported (9). In a recent retrospective cohort study, patients who were hospitalized during HCV treatment and missed more than 10 doses of their DAA therapy, had lower SVR rates than those with fewer missed doses (9). As HCV treatment courses continue to be shortened missing as few as 5 days of therapy could significantly increase a patients risk of treatment failure (9,20).
In addition to therapeutic interruptions, the majority of patients experienced potentially clinically significant drug interactions between their DAA therapy and other concurrent medications. More than half of all pharmacological interactions were the result of medications initiated in hospital. The most common interactions involved acid-suppressing therapy which are commonly prescribed in both the inpatient and outpatient setting. The prevalence of acid suppressing therapy is high in North America. In British Columbia, 3% of all citizens were prescribed a PPI at least once in 2019 (21). Prevalence of PPI use in patients with HCV is much higher with nearly 40% of United States Medicaid patients being prescribed a PPI at least once in 2011 (22). This rate is similar to the overall rate of PPI prescribing in hospitalized patients (23). Prescribing information recommends avoiding PPIs in patients taking NS5A inhibitor containing regimens unless absolutely necessary, and if necessary, a reduced dosage or revised dosing schedule is recommended (17,24,25).
The results of our study also highlighted a paucity of knowledge in identifying and managing drug interactions with DAA regimens. Clinicians failed to identify potentially significant, and contraindicated drug interactions between DAAs and other therapies. This may have been in part due to a lack of familiarity with DAA therapies as well as increased number and complexity of medications prescribed to many hospitalized patients. In the outpatient setting, comprehensive assessment and management of drug interactions with DAA therapy requires approximately 30 minutes of time from a clinical pharmacist with experience in treating HCV (26). Inpatient clinical pharmacists with limited expertise in HCV pharmacotherapy can be expected to take longer to perform an equivalent assessment. Such onerous time commitment may be difficult to incorporate into clinical pharmacists’ daily workflow. Information technology failures may have also contributed to missed drug interactions. Since DAA regimens are non-formulary in our hospital, most were entered onto the medication administration record using free text option which would preclude them from automated drug interaction checks (27). As such, pharmacists without intimate knowledge of DAA therapies would have to manually perform drug interaction checks to identify suboptimal, and in some cases, dangerous combinations.
Despite frequent therapy interruptions, and prevalent drug interactions, all patients who survived to discharge achieved SVR24. No clinically significant complications related to DAA therapy or drug interactions with DAAs were documented. Of note, patients included in this study were primarily initiated on HCV therapy by gastroenterologists and infectious diseases specialists in consultation an expert in DAA pharmacotherapy.
This study has several limitations including its retrospective cross-sectional nature and small sample size. There were only 57 episodes of hospitalization in 54 patients while receiving DAA treatment over 3.5 years. Although almost all patients achieved SVR24 despite clinically significant drug interactions and therapy interruptions, no conclusions can be drawn about the safety of such inadvertent events. For example, it is possible that complications due to unidentified interactions with DAAs such as rhabdomyolysis, hypotension, and sedation were simply missed due to retrospective data collection. Furthermore, if a DAA therapy course was never restarted in hospital, we would have simply missed including that patients in our study as we used EHR to generate a list of patients who were ordered DAA regimens while in hospital. This could dramatically change the proportion of patients achieving SVR given the small number of patients included in our study. Also, we were unable to ascertain the initial start date of DAA therapy in our patients; as such, some patients may have already effectively cleared their HCV infection prior to admission. The majority of patients were treated with early generation DAAs that did not contain HCV protease inhibitors. This could impact the generalizability of our results to the commonly used DAA combinations currently used. Protease inhibitors interact with more medications than the NS5A/NS5B inhibitors due to their ability to modulate the cytochrome P450 system which is involved in the metabolism of more than 50% of currently marketed medications (28). HCV protease inhibitors have been identified as an independent risk factor for DAA drug interactions (29). Further real world analysis is needed with other combinations prone to drug interactions, and in patients prescribed treatment courses of less than 12 weeks (30). Important strengths of this study include that it is the first real world study to examine the extent of treatment interruptions and drug interactions in hospitalized patients, including patients not being followed by physician or pharmacist with expertise in HCV pharmacotherapy.
Conclusions
The results of our study highlight that inadvertent treatment interruptions and drug interactions are common in patients admitted to the hospital while taking DAA regimens. Although modern DAA therapies are potent and effective antivirals with high rates of treatment success, their provision requires expert knowledge in managing drug interactions and near perfect compliance. As clinicians and public health agencies reorient priorities post-COVID, a shift in DAA prescribing from specialized clinics to primary care offices coupled with broadened eligibility criteria will lead to increased number of patients admitted to hospital while on DAA therapy. Based on our results, there is a general lack of familiarity with DAA regimens, their common drug interactions, and how to best mitigate such interactions. Clinician education, implementation of information technology safeguards such as automated drug interaction checkers and clinical decision support tools can be effectively utilized to optimize care for patients admitted to hospitals while taking HCV DAA therapy. Availability of lower cost generic DAAs and their addition to the hospital formularies will also remove supply chain barriers facilitating timely access to these medications.
Acknowledgments
The authors would like to thank Steven Du and Jo-Ann Ford for their assistance in data acquisition.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-80/rc
Data Sharing Statement: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-80/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-80/coif). EMY serves as an unpaid Associate Editor-in-Chief of AME Medical Journal from June 2017 to December 2024. TH serves as an unpaid Associate Editor-in-Chief of AME Medical Journal from November 2018 to November 2024. RCW has received an honorarium from the Canadian Society of Hospital Pharmacists for CME events. MK has received contract and grant funding from Siemens unrelated to this work. EMY has been an investigator of clinical trials sponsored by Gilead Sciences, Merck Inc, AbbVie, Madrigal, Intercept, Allergan, Genfit, Pfizer, Celegene, and Novo Nordisk. He has received honoraria for CME lectures and advisory board participation by Intercept Canada and Merck Canada. He holds leadership roles on the following boards and associations: Canadian Association for the Study of Liver, Canadian Liver Foundation, Royal Canadian Legion, TB Vets Society, Canadian Society for Transplantation, Vancouver Medical Dental Allied Staff Association, and Vancouver Physician Staff Association. TH has received an investigator-initiated research grant from Paladin Labs Inc. The other author has no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Hepatitis C [Internet]. 2021 [cited 2022 Feb 12]. p. 1–8. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c#:~:text=Globally%2C an estimated 58 million,carcinoma (primary liver cancer)
- Compagnoni S, Bruno EM, Madonia G, et al. Direct antiviral agents in hepatitis C virus related liver disease: Don't count the chickens before they're hatched. World J Gastroenterol 2021;27:2771-83. [Crossref] [PubMed]
- Radley A, Robinson E, Aspinall EJ, et al. A systematic review and meta-analysis of community and primary-care-based hepatitis C testing and treatment services that employ direct acting antiviral drug treatments. BMC Health Serv Res 2019;19:765. [Crossref] [PubMed]
- Shah H, Bilodeau M, Burak KW, et al. The management of chronic hepatitis C: 2018 guideline update from the Canadian Association for the Study of the Liver. CMAJ 2018;190:E677-87. [Crossref] [PubMed]
- Tadrous M, Mason K, Dodd Z, et al. Prescribing trends in direct-acting antivirals for the treatment of hepatitis C in Ontario, Canada. Can Liver J 2021;4:51-8. [Crossref] [PubMed]
- Omland LH, Osler M, Jepsen P, et al. Socioeconomic status in HCV infected patients - risk and prognosis. Clin Epidemiol 2013;5:163-72. [Crossref] [PubMed]
- Recommendations for Direct-Acting Antiviral Agents for Chronic Hepatitis C Genotype 1 [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2014 Oct.
- Pacific Blue Cross. Pharmacy Compass [Internet]. [cited 2022 Jun 26]. Available online: https://www.pac.bluecross.ca/pharmacycompass
- Gentene AJ, Bell AM, Pence A, et al. Sustained Virologic Response of Patients Hospitalized Compared With Those Not Hospitalized During Treatment for Hepatitis C Virus With Direct-Acting Antivirals. Ann Pharmacother 2021;55:565-74. [Crossref] [PubMed]
- Foster GR, Dore GJ, Wang S, et al. Glecaprevir/pibrentasvir in patients with chronic HCV and recent drug use: An integrated analysis of 7 phase III studies. Drug Alcohol Depend 2019;194:487-94. [Crossref] [PubMed]
- Mason K, Dodd Z, Guyton M, et al. Understanding real-world adherence in the directly acting antiviral era: A prospective evaluation of adherence among people with a history of drug use at a community-based program in Toronto, Canada. Int J Drug Policy 2017;47:202-8. [Crossref] [PubMed]
- Serper M, Evon DM, Stewart PW, et al. Medication Non-adherence in a Prospective, Multi-center Cohort Treated with Hepatitis C Direct-Acting Antivirals. J Gen Intern Med 2020;35:1011-20. [Crossref] [PubMed]
- Sundberg I, Lannergård A, Ramklint M, et al. Direct-acting antiviral treatment in real world patients with hepatitis C not associated with psychiatric side effects: a prospective observational study. BMC Psychiatry 2018;18:157. [Crossref] [PubMed]
- de Oliveira LM, Diel JDAC, Nunes A, et al. Prevalence of drug interactions in hospitalised elderly patients: a systematic review. Eur J Hosp Pharm 2021;28:4-9. [Crossref] [PubMed]
- Smolders EJ, Berden FA, de Kanter CT, et al. The majority of hepatitis C patients treated with direct acting antivirals are at risk for relevant drug-drug interactions. United European Gastroenterol J 2017;5:648-57. [Crossref] [PubMed]
- Kondili LA, Gaeta GB, Ieluzzi D, et al. Real-life data on potential drug-drug interactions in patients with chronic hepatitis C viral infection undergoing antiviral therapy with interferon-free DAAs in the PITER Cohort Study. PLoS One 2017;12:e0172159. [Crossref] [PubMed]
- Hong J, Wright RC, Partovi N, et al. Review of Clinically Relevant Drug Interactions with Next Generation Hepatitis C Direct-acting Antiviral Agents. J Clin Transl Hepatol 2020;8:322-35. [Crossref] [PubMed]
- Liverpool U of. HEP Drug Interactions [Internet]. [cited 2022 Feb 12]. Available online: https://www.hep-druginteractions.org/checker
- Inc. L. Lexi-Interact [Internet]. [cited 2022 Feb 12]. Available online: https://online.lexi.com/lco/action/interact
- Brown RS Jr, Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J Hepatol 2020;72:441-9. [Crossref] [PubMed]
- Initiative TT. Trends in utilization of proton pump inhibitors in British Columbia. Therapeutics Newsletter. 2019.
- Pesa JA, Wang L, Yuce H, et al. Proton-Pump Inhibitor Utilization Among Patients With Hepatitis C Virus. Value Heal 2016;19:A213.
- Durand C, Willett KC, Desilets AR. Proton Pump Inhibitor use in Hospitalized Patients: Is Overutilization Becoming a Problem? Clin Med Insights Gastroenterol 2012;5:65-76. [Crossref] [PubMed]
- Gilead Sciences. EPCLUSA® (sofosbuvir/velpatasvir) product monograph [Internet]. 2019 [cited 2020 Jan 19]. Available online: http://www.gilead.ca/application/files/9615/6925/4628/Epclusa_English_PM_e186388-GS-005.pdf
- German P, Mathias A, Brainard D, et al. Clinical Pharmacokinetics and Pharmacodynamics of Ledipasvir/Sofosbuvir, a Fixed-Dose Combination Tablet for the Treatment of Hepatitis C. Clin Pharmacokinet 2016;55:1337-51. [Crossref] [PubMed]
- Langness JA, Nguyen M, Wieland A, et al. Optimizing hepatitis C virus treatment through pharmacist interventions: Identification and management of drug-drug interactions. World J Gastroenterol 2017;23:1618-26. [Crossref] [PubMed]
- Flockhart D, Honig P, Yasuda S, et al. Preventable Adverse Drug Reactions: A Focus on Drug Interactions [Internet]. 2018 [cited 2022 Aug 3]. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/preventable-adverse-drug-reactions-focus-drug-interactions
- Cerny MA. Prevalence of Non-Cytochrome P450-Mediated Metabolism in Food and Drug Administration-Approved Oral and Intravenous Drugs: 2006-2015. Drug Metab Dispos 2016;44:1246-52. [Crossref] [PubMed]
- Margusino-Framiñán L, Cid-Silva P, Giménez-Arufe V, et al. Influence of drug-drug interactions on effectiveness and safety of direct-acting antivirals against hepatitis C virus. Eur J Hosp Pharm 2021;28:16-21. [Crossref] [PubMed]
- AbbVie. MAVIRET® (glecaprevir/pibrentasvir) product monograph [Internet]. 2020 [cited 2020 Jan 19]. Available online: https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=95591
Cite this article as: Wright RC, McGinnis M, Krajden M, Yoshida EM, Hussaini T. Therapy interruptions and drug interactions during acute hospitalization in patients taking direct acting antivirals for chronic hepatitis C virus infection: a retrospective cross-sectional study. AME Med J 2023;8:2.

