
A narrative review of precision medicine in metastatic renal cell carcinoma
Introduction
Background
In 2020, new cases of renal cell carcinoma (RCC) were diagnosed about 430,000 patients all over the world (1). Clinically, surgery is the first choice for RCC without metastasis. In addition, approximately 30% of new RCC cases are metastatic RCC (mRCC), and these patients mainly receives systematic therapy (2). Conventionally, treatment of mRCC consists of interferon-alpha (IFNα) therapy (3,4), which leads to poor therapeutic outcomes.
Rationale and knowledge gap
However, with the development of tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin (mTOR), outcomes for mRCC have improved dramatically; with these drugs, there have been significant improvements in overall survival (OS) (5-7). The prognosis for mRCC is generally based on the International mRCC Database Consortium (IMDC). In the TKIs era, 2-year OS was 81.6% (favourable risk), 48.7% (intermediate risk), 23.4% (poor risk) (8). Since then, several TKIs have been developed to improve treatment outcomes for mRCC, with an increasing number of treatment options, including first-, second-, and third-line treatments (9,10).
Objective
Immune checkpoints suppress the antitumor immune response; antibody drugs against anti-programmed cell death 1 (PD-1), programmed cell death-ligand 1 (PD-L1), or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) were developed, and their efficacy was confirmed by randomized controlled trials (RCTs). CheckMate 025 compared nivolumab, a PD-1 antibody drug, to everolimus in patients with mRCC. This study included 821 patients with advanced renal cancer. Nivolumab showed significant reduction of mRCC tumor size and prolonged OS (11). Since then, immune-oncology (IO) treatments have changed the treatment of cancer. The treatment options for mRCC have increased with the advent of various IO drugs. For these reasons, the most widely used first-line treatment of mRCC is dual IO drugs or a combination of IO-TKIs. However, it has been difficult to decide which drug is the best choice with regard to therapeutic effects, adverse events (AEs), and immune-related adverse events (irAEs).
The difference between our narrative and other reviews discussed we reported not only untreated clear cell mRCC but also untreated non-clear cell mRCC (12,13). In addition, we discuss the optimal treatment modality for patients with mRCC based on the currently under developed drugs, using analysis of polybromo 1 (PBRM1), BRCA1-associated protein 1 (BAP1), plasma cell-free DNAs (cfDNA), repertoire analysis of T-cell receptors (TCR), the gut microbiome and so on. Especially, we discuss gut microbiome as a various potential to treatment of mRCC because it associated with cancer immune response. We present this article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-83/rc).
Methods
A literature search was performed on PubMed for articles published from January 1998 to December 2022, focusing on articles relevant to mRCC and first-line mRCC therapy using IO drugs. The keywords, “mRCC”, “gene expression”, “precision medicine”, “gut microbiome”, and “antibiotics” from clinical studies were used (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 2022.12.31 |
| Databases and other sources searched | PubMed |
| Search terms | “metastatic renal cell carcinoma” [MeSH] |
| “gene expression” [MeSH] | |
| “precision medicine” [MeSH] | |
| “gut microbiome” [MeSH] | |
| “antibiotics” [MeSH] | |
| Timeframe | January 1998 to December 2022 |
| Inclusion and exclusion criteria | Inclusion: original papers and reviews in English about themes such as renal cell carcinoma, gene expression, precision medicine, gut microbiome and antibiotics |
| Exclusion: articles which we considered with low reliability | |
| Selection process | It was conducted by K Sugiomo, K Fujita and other co-authors |
First-line mRCC therapy using dual IO drugs or IO-TKIs drugs
Several guide lines for mRCC patients recommend combination IO therapies or IO-TKIs therapies as a first-line treatment. RCTs have shown that these combination therapies improve OS and progression-free survival (PFS). We summarized the pivotal the trials of mRCC studies regarding outcomes, AEs, and irAEs (14-25) (Table 2).
Table 2
| Variables | Regimen | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JAVELIN Renal 101 (14,15) | KEYNOTE-426 (16,17) | Check Mate 214 (18-21) | Check Mate 9ER (22) | CLEAR (23) | IMmotion151 (24,25) | |||||||||||||
| Ave + Axi | Sun | Pem + Axi | Sun | Nivo + Ipi | Sun | Nivo + Cabo | Sun | Pem + Len | Len + Eve | Sun | Atz + Bev | Sun | ||||||
| N | 442 | 444 | 432 | 429 | 425 | 422 | 323 | 328 | 355 | 357 | 357 | 454 | 461 | |||||
| Median age [range] (years) | 62 [29–83] | 61 [27–88] | 62 [30–89] | 61 [26–90] | 62 [26–85] | 61 [21–85] | 62 [29–90] | 61 [28–86] | 64 [34–88] | 62 [32–86] | 61 [29–82] | 62 [24–88] | 60 [18–84] | |||||
| Sex (male/female), n (%) | 316 (71.5)/126 (28.5) |
344 (77.5)/100 (22.5) |
308 (71.3)/124 (28.7) |
320 (74.6)/109 (25.4) |
314 (73.9)/111 (26.1) |
301 (71.3)/121 (28.7) |
249 (77.1)/74 (22.9) |
232 (70.7)/96 (29.3) |
255 (71.8)/100 (28.2) |
266 (74.5)/91 (25.5) |
275 (77.0)/82 (23.0) |
317 (70.0)/137 (30.0) |
352 (76.4)/109 (23.6) |
|||||
| IMDC risk, % | ||||||||||||||||||
| Favorable | 21.3 | 21.6 | 31.9 | 30.5 | – | – | 22.9 | 22.0 | 31.0 | 31.9 | 34.7 | – | – | |||||
| Intermediate | 61.3 | 62.20 | 55.1 | 57.3 | 79.0 | 79.0 | 58.2 | 57.3 | 59.2 | 54.6 | 53.8 | – | – | |||||
| Poor | 16.3 | 16.0 | 13.0 | 12.1 | 21.0 | 21.0 | 18.9 | 20.7 | 9.3 | 11.8 | 10.4 | – | – | |||||
| Not reported | 1.1 | 0.2 | – | – | – | – | – | – | 0.6 | 1.7 | 1.1 | – | – | |||||
| PD-L1 expression ≥1%, n (%) | 270 (61.1) | 290 (65.3) | 243 (59.3) | 254 (61.7) | 100 (Jeny26) | 114 (Jeny29) | 83 (25.7) | 83 (25.3) | 107 (30.1) | 116 (32.5) | 119 (33.3) | 178 (39.2) | 184 (40.0) | |||||
| Median follow-up (months) | 13.0 | 30.6 | 67.7 | 18.1 | 27.0 | – | ||||||||||||
| OS (months) | ||||||||||||||||||
| Median (95% CI) | NR (30.0–NE) | NR (27.4–NE) | NR | 35.7 | 47.0 (35.5–57.4) | 26.6 (22.1–35.5) | NR | NR (22.6–NE) | NR (33.6–NE) | NR | NR | 36.1 (31.5–42.3) | 35.3 (28.6-42.1) | |||||
| HR (95% CI); P value | 0.80 (0.616–1.027); 0.0392 | 0.68 (0.55–0.85); 0.0003 | 0.68 (0.58–0.81); <0.0001 | 0.60 (98.89% CI: 0.40–0.89); 0.001 | Pem + Len vs. Sun: 0.66 (0.49–0.88); 0.005 | 0.91 (0.76–1.08); 0.27 | ||||||||||||
| Len + Eve vs. Sun: 1.15 (0.88–1.50); 0.30 | ||||||||||||||||||
| PFS (months) | ||||||||||||||||||
| Median (95% CI) | 13.3 (11.1–15.3) | 8.0 (6.7–9.8) | 15.4 | 11.1 | 11.6 (8.4–16.5) | 8.3 (7.0–10.4) | 16.6 (12.5–24.9) | 8.3 (7.0–9.7) | 23.9 (20.8–27.7) | 14.7 (11.1–16.7) | 9.2 (6.0–11.0) | – | – | |||||
| HR (95% CI); P value | 0.69 (0.574–0.825); <0.0001 | 0.71 (0.60–0.84); <0.0001 | 0.73 (0.61–0.87); 0.0004 | 0.51 (0.41–0.64); <0.001 | Pem + Len vs. Sun: 0.39 (0.32–0.49); <0.001 | – | ||||||||||||
| Len + Eve vs. Sun: 0.65 (0.53–0.80); <0.001 | ||||||||||||||||||
| ORR (95% CI), % | 52.5 (47.7–57.2) | 27.3 (23.2–31.6) | 60.4 | 39.6 | 41.9 | 26.8 | 55.7 (50.1–61.2) | 27.1 (22.4–32.3) | 71.0 (66.3–75.7) | 53.5 (48.3–58.7) | 36.1 (31.2–41.1) | – | – | |||||
| DCR, % | 80.8 | 70.9 | 83.3 | 72.8 | 72.7 | 71.1 | 87.9 | 69.2 | 90.1 | 87.1 | 74.2 | – | – | |||||
| Best overall response, n (%) | ||||||||||||||||||
| Complete response | 17 (3.8) | 9 (2.0) | 38 (8.8) | 13 (3.0) | 44 (10.4) | 6 (1.4) | 26 (8.0) | 15 (4.6) | 57 (16.1) | 35 (9.8) | 15 (4.2) | – | – | |||||
| Partial response | 215 (48.6) | 112 (25.2) | 222 (51.4) | 158 (36.8) | 134 (31.5) | 107 (25.4) | 154 (47.7) | 74 (22.6) | 195 (54.9) | 156 (43.7) | 114 (31.9) | – | – | |||||
| Stable disease | 125 (28.3) | 194 (43.7) | 100 (23.1) | 150 (35.0) | 131 (30.8) | 187 (44.3) | 104 (32.2) | 138 (42.1) | 68 (19.2) | 120 (33.6) | 136 (38.1) | – | – | |||||
| Progressive disease | 55 (12.4) | 86 (19.4) | 49 (11.3) | 74 (17.2) | 82 (19.3) | 71 (16.8) | 18 (5.6) | 45 (13.7) | 19 (5.4) | 26 (7.3) | 50 (14.0) | – | – | |||||
| Unknown | 30 (6.8) | 43 (9.7) | 23 (5.3) | 34 (7.9) | 34 (8.0) | 51 (12.1) | 21 (6.5) | 56 (17.1) | 16 (4.5) | 20 (5.6) | 42 (11.8) | – | – | |||||
| Toxicities, % (all grades) | 99.5 | 97.3 | 96.3 | 97.6 | 94.0 | 97.4 | 99.7 | 99.1 | 99.7 | 99.7 | 98.5 | 98.0 | 98.9 | |||||
| Events, % | G3,4: 71.2 | G3,4: 71.5 | G3–5: 67.8 | G3–5: 63.8 | G3,4: 47.9 | G3,4: 64.1 | G3,4: 75.3 | G3,4: 70.6 | G3,4: 82.4 | G3,4: 83.1 | G3,4: 71.8 | G3,4: 45.5 | – | |||||
Atz, atezolizumab; Ave, avelumab; Axi, axitinib; Bev, bevacizumab; Cabo, cabozantinib; CI, confidence interval; DCR, disease control rate; Eve, everolimus; HR, hazard ratio; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IO, immune-oncology; Ipi, ipilimumab; Len, lenvatinib; mRCC, metastatic renal cell carcinoma; NE, could not be estimated; Nivo, nivolumab; NR, not reached; ORR, objective response rate; OS, overall survival; PD-L1, programmed cell death-ligand 1; Pem, pembrolizumab; PFS, progression-free survival; Sun, sunitinib; TKIs, tyrosine kinase inhibitors.
PD-L1 expressed on the surface of cancer cells allows them to escape immune cells. Nivolumab promotes immune cells to attack cancer cells by suppressing PD-1 (26). Nivolumab is the first anti-PD-1 antibody to be indicated for mRCC from CheckMate 025 results (11). CTLA-4 is up-regulated shortly after T-cell activation and initiates negative regulatory signaling on T-cells during ligation with B7 costimulatory molecules expressed by antigen-presenting cells (27). Ipilimumab is a monoclonal antibody targeting CTLA-4 that blocks inhibitory signals to cytotoxic T-lymphocytes. CheckMate 214, comparing nivolumab + ipilimumab versus sunitinib, showed OS improvement for intermediate/poor (I/P)-risk untreated clear cell mRCC with nivolumab + ipilimumab (18,19). This study showed no significant differences in OS between the favorable risk groups. However, these combination therapies show a durable effect of I/P-risk untreated clear cell mRCC by inducing cancer immunity. In these groups, nivolumab + ipilimumab had significant benefits over sunitinib with respect to PFS [hazard ratio (HR) =0.68; 95% confidence interval (CI): 0.58–0.81] and OS (HR =0.73; 95% CI: 0.61–0.87). An objective response occurred in 41.9% of patients treated with nivolumab + ipilimumab and 26.8% of patients treated with sunitinib. In the pathological analysis of CheckMate 214, nivolumab + ipilimumab is also effective with sarcomatoid mRCC in first-line treatment (PFS: HR =0.54; 95% CI: 0.33–0.86 and OS: HR =0.45; 95% CI: 0.30–0.70) (28). In other study, CheckMate 920 study showed these combination therapies is safety and efficacy in patients with non-clear cell mRCC (29). The results showed median PFS was 3.7 (95% CI: 2.7–4.6) months, median OS was 21.2 (95% CI: 16.6–estimable) months.
Axitinib is a potent, and highly selective inhibitor of vascular endothelial cell growth factor receptor-1, -2, -3 (VEGFR-1, -2, and -3). Excessive production VEGFR suppresses immune cell function. The tumor microenvironment consists of tumor cells, blood vessels, and extracellular matrix. The tumor microenvironment affects the efficacy of IO drugs, and the combination of axitinib with IO drugs is considered to improve the microenvironment favoring IO drugs (30,31). Comparing the effects of pembrolizumab, PD-1 inhibitor, + axitinib or sunitinib on first-line mRCC therapies, combination therapy has superior clinical outcomes.
Cabozantinib, which has been used to treat mRCC and hepatocellular carcinoma (22,32). Cabozantinib is a multikinase inhibitor that targets receptor tyrosine kinases, including VEGFR-2, the hepatocyte growth factor receptor (MET), and the growth arrest-specific 6 (GAS6) receptor (AXL) (33,34). Nivolumab + cabozantinib had significant benefits over sunitinib with respect to PFS (HR =0.51; 95% CI: 0.41–0.64) and OS (HR =0.60; 95% CI: 0.40–0.89) with previously untreated mRCC (20). An objective response occurred in 55.7% of patients treated with nivolumab + cabozantinib and 27.1% of patients treated with sunitinib. In addition, subgroup analysis showed that combination therapy of nivolumab + cabozantinib was highly effective in cases with bone metastasis, and showed superior response rates to sunitinib. Moreover, combination therapy led to improved quality of life compared to sunitinib monotherapy. In other pathological analysis, cabozantinib + nivolumab showed promising efficacy in the patients with papillary, unclassified, translocation-associated RCC and chromophobe RCC in a phase II trial (35).
Lenvatinib is a multi-kinase inhibitor that, inhibits VEGFRs and fibroblast growth factor receptors (FGFR). Moreover, by inhibiting tyrosine kinase receptors such as rearranged during transfection (RET) and FGFR in cancer cells, lenvatinib suppresses signal transduction in cancer growth (36). Another study using mouse and RCC cell lines showed that lenvatinib + anti-PD-1 antibody strengthened the antitumor activity (37). Lenvatinib blocked FGFR signaling in cancer cells, and restored the expression of interferon-gamma (IFNγ) target molecules that were suppressed by FGFR signaling. These mechanisms provide strong anti-tumor activity by combination therapy with lenvatinib and PD-1 inhibitors. Activation of the IFNγ/Janus kinase (JAK)/signal transducers and activator of transcription (STAT) pathway increases the expression of PD-L1 and chemokines to suppress cancer immunology. Therefore, this combination therapy helps tumor cells to avoid the host immune system (34). In a CLEAR trial comparing pembrolizumab + lenvatinib or sunitinib to advanced RCC, pembrolizumab + lenvatinib was associated with significantly longer PFS (HR =0.39; 95% CI: 0.32–0.49) and OS (HR =0.66; 95% CI: 0.49–0.88) than sunitinib alone. However, this combination therapy resulted in grade 3 or higher AEs (e.g., hypertension or diarrhea) in at least 10% of patients (23). These combination therapies are very effective for the IMDC poor risk group in the subgroup analysis of PFS because it is useful with large tumor volume patients. From these results, there also hoped to be effective for mRCC in many various pathological types.
PD-L1 is expressed on RCC cells, and inhibits the activation of cytotoxic T cells that can recognize and attack cancer cells (38). Avelumab inhibits PD-1/PD-L1 compound formation and promotes the immune response (14,39). In a JAVELIN Renal 101 clinical trial comparing avelumab + axitinib or sunitinib for patients with mRCC first-line treatment, avelumab + axitinib was associated with a significantly longer PFS than sunitinib (HR =0.69; 95% CI: 0.574–0.825). It has been suggested that almost half of patients do not benefit from this regimen because of its efficacy and the resulting toxicity. Therefore, the identification of factors associated with treatment efficacy remains a problem.
Atezolizumab, a PD-L1 inhibitor, has pharmacological effects similar to those of avelumab (37). Bevacizumab is a monoclonal antibody for VEGF-A. It suppresses angiogenesis, tumor growth, and metastasis (40). In an IMmotion151 trial, atezolizumab + bevacizumab or sunitinib was compared as first-line treatment for treatment-naïve patients with mRCC. Combination therapy showed as improvement in PFS, but no improvement in OS (HR =0.91; 95% CI: 0.76–1.08) in patients with previously untreated mRCC (24,25).
The type of AEs differs depending on the drug combination used. Including TKIs regimens of any grade resulted in hypertension in approximately 50% of patients, hypothyroidism in about 25–45%, and fatigue in about 30%. Diarrhea should be noted when using avelumab + axitinib, nivolumab + cabozantinib, and pembrolizumab + lenvatinib because the rate of grade ≥3 AE was ≥7% in these first-line treatment options.
All combination therapies showed excellent outcomes. For these reasons, therapeutic efficacy for mRCC has dramatically improved (41). The combination therapy of nivolumab + ipilimumab or pembrolizumab + lenvatinib may be the good option for IMDC poor risk group; however, these trials did not directly compare each therapy for the first-line treatment of mRCC. Therefore, combination therapies should be chosen based on treatment efficacy, patient risk profile, and tolerance to each treatment.
Recently, many DNA-based biomarkers such as tumor mutational burden (TMB), tumor indel burden (TIB), human leukocyte antigen (HLA) have been associated with response to immune checkpoint inhibitors (ICI). Biomarker analysis of CheckMate 214 showed that low TIB was associated with PFS, but not OS (42). In the future, biomarker analysis helped to provide an insight into which mRCC patients would benefit from these combination therapies.
Precision medicine based on T-cell related gene expression, PBRM1, and BAP1
The development of next-generation sequencing has enabled us to understand the details of gene expression and mutations.
PBMR1 and BAP1 are tumor suppressor genes located on the short arm of chromosome 3. Recent studies showed that PBMR1 and BAP1 mutations are involved in chromatin regulation (43,44), and that these mutated genes are responsible for the pathogenesis of RCC. The predictive roles of these mutated genes have been reported. Mutational biomarkers reported, the study using targeted sequencing of 341 cancer genes was performed on tumor samples from 258 patients with mRCC. For the first-line treatment of mRCC, everolimus and sunitinib showed good efficacy rates in patients with PBRM1 mutations, but not in patients with BAP1 mutations (45). BAP1 gene mutations and the expression of intrinsic endogenous retroviruses (ERVs) are related to each other. ERVs are involved in the immune induction of cancer. ERV expression is associated with local immune checkpoint activation (ICA) and the response to immune checkpoint blockade (ICB) (46). For these reasons, IO is effective when ERV expression exists in clear cell RCC.
Analysis of PBRM1 and BAP1 gene mutations could help in selecting treatment options for patients with mRCC.
cfDNAs are degraded DNA fragments (50–200 bp) released into the blood plasma from tumor cells as well as normal cells. cfDNA could be a surrogate marker for multiple cancers, and can be used for diagnosis, prognosis, and monitoring because of tumor specific alterations in cfDNA (47).
Analysis of plasma cfDNA is less invasive than that of needle biopsy specimens. Mutated PBRM1 and BAP1 could be detected by the analysis of plasma cfDNA (48-50), which could lead to precision medicine.
Comprehensive molecular characterization
Omic-based medicine integrates comprehensive molecular information to improve precision medicine. Motzer et al. reported integrated multi-omics analyses, leading to the identification of robust molecular subtypes in 823 tumors from patients with advanced RCC, including 134 tumors with sarcomatoid features (51). This trial compared atezolizumab + bevacizumab with sunitinib as the first-line treatment for patients with mRCC (24). Unsupervised transcriptomic analysis revealed seven molecular subsets with angiogenesis, immunity, cell-cycle, metabolism, and stromal programs. Angiogenesis-enriched patients demonstrated superior prognosis in both the atezolizumab + bevacizumab and sunitinib groups because of the presence of an angiogenesis inhibitor. Atezolizumab + bevacizumab showed clinical benefits in patients with high T-effectors and/or cell-cycle transcription. These findings can be applied to stratify patients based on molecular subsets, improve the clinical outcomes of mRCC by selecting checkpoint blockade or antiangiogenic therapy alone, and lead to personalized therapies for mRCC.
Radiogenomic
The therapeutic effects of VEGFR for patients with mRCC was determined using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). DCE-MRI is a multiparametric MRI, that can evaluate the permeability of blood vessels in tumor microenvironments (52). The pharmacodynamic effects of VEGFR TKIs were evaluated by assessing changes in contrast-enhancement parameters of metastatic liver lesions using DCE-MRI in patients with colon cancer. DCE-MRI parameters are correlated with pharmacological responses, and DCE-MRI can monitor vascular changes (53). Another prospective study included 49 patients with clear cell RCC who underwent DCE-MRI prior to nephrectomy. The surgical specimens were sectioned to match the MRI acquisition planes. RNA data from tumor sampling were correlated with the percent enhancement on DCE-MRI. DCE-MRI findings suggested the method can be used to determine associated gene expression of angiogenesis, IO cells, and the response of metastatic lesions. DCE-MRI can predict the response of patients to VEGFR-TKI therapy. Hence, DCE-MRI has the highest potential among comprehensive imaging-based approaches (54).
Repertoire analysis of TCR
TCR analysis is a potential biomarker for treatment outcomes of immune ICI. To recognize a large variety of antigens, humans have a huge diversity of TCR repertoires through somatic recombination of TCR chains. Advances in next-generation sequencing technologies, coupled with powerful novel bioinformatics tools, allow quantitative and reproducible characterization of TCR repertoires in tumor and blood samples from an increasing number of patients with a variety of solid cancers. The analysis of TCR repertoires can be used to detect malignant lymphoma and, leukemia cells, and to evaluate the efficacy of ICIs (55).
Analysis of the TCR repertoire in peripheral blood samples predicts the efficacy of PD-1 monotherapy (nivolumab). Patients with a decreased diversity index (DI) of TCR repertoires one month after treatment have a better prognosis compared to patients with increased DI (56). The TCR repertoire and level of PD-1 expression in peripheral blood have the potential to provide predictive biomarkers for the therapeutic efficacy of ICIs due to the response to anti-PD-1 monotherapy after treatment initiation in patients with mRCC. TCR repertoire analysis could be a useful tool for determining the treatment efficiency of IO therapies.
Gut microbiome
Recently, it has been proven that the gut microbiome is involved in the pathogenesis of inflammatory bowel disease, obesity, diabetes, cancer, autism, atherosclerosis, etc. (57-60). The gut microbiome is also associated with immune regulations (61). The gut microbiome was affected by diet, residential area, and race (62,63).
The influence of antibiotics in patients with advanced non-small cell lung cancer (NSCLC), RCC, and urothelial carcinoma who received IO drugs was recently reported. Antibiotics can significantly alter the gut microbiome. A total of 249 patients were analyzed; 69 patients were prescribed antibiotics from two months prior up to one month after the first administration of IO. PFS and OS were significantly shorter in the antibiotic treated group than in the treated group (64).
In another study, the gut microbiomes of 69 patients with mRCC who underwent nivolumab treatment were analyzed. Therapeutic effects were examined based on the presence or absence of a recent history of antibiotic administration. Among the 69 patients, 11 (16%) received antibiotics, and 58 (84%) did not. The group that received antibiotics had a lower objective response rate than the non-antibiotic group. Akkermansia muciniphila and Bacteroides salyersiae are prevalent in the feces of the responder group (65). We summarized the three studies for the gut microbiome in urological cancer (Table 3). The effect of antibiotic use was also studied in patients with mRCC who received nivolumab + ipilimumab. There were no significant differences between patients with a history of antibiotic use within 3 months compared to patients without a history of antibiotic use in terms of PFS and OS. However, the patients without a history of antibiotic use had a longer OS compared with patients with a history [median OS: non-antibiotics: not reached (NR) (95% CI: 18.7 to NR) months vs. antibiotics: NR (95% CI: 16.4 to NR) months, P=0.270] (66). Gut microbiota directly affects the immune cells in the gut, or the metabolites from gut microbiota, that can enter the systemic circulation and affect immune cells.
Table 3
| Cancer | Endpoint | Patients (N) | Clinical outcome | Reference |
|---|---|---|---|---|
| NSCLC | OS | 140 | Median OS: non-ATBs 15.3 mo vs. ATBs 8.3 mo, P=0.001 | (64) |
| RCC | PS | 67 | Median OS: non-ATBs 7.4 mo vs. ATBs 4.3 mo, P=0.012 | (64) |
| mRCC | ORR | 69 | Non-ATBs 52% vs. ATBs 18% | (65) |
| mRCC | PFS, OS | 72 | Non-ATBs vs. ATBs: PFS, P=0.272; OS, P=0.270 | (66) |
ATBs, antibiotics; IO, immune-oncology; mRCC, metastatic renal cell carcinoma; mo, month; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; RCC, renal cell carcinoma.
The relationship between microbiota and the therapeutic effects of IO drugs is summarized in Table 2. As these studies had a small number of cases, it is necessary to increase the number of cases to prove their credibility. Analysis of the gut microbiome might be a promising method for predicting treatment efficacy in patients with mRCC.
The limitation of our narrative review is no consensus on the optimal treatment for such patients because there are no phase III clinical studies based on biomarkers for mRCC treatment. In the near future, we believe that these biomarkers will lead to precision medicine in the treatment of mRCC by being analyzed carefully and strategically.
Conclusions
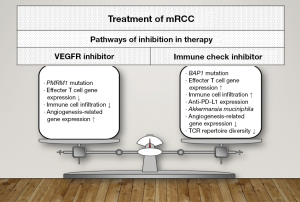
The standard first-line therapies for patients with mRCC are combination IO therapies or IO-TKIs drugs. PBRM1, BAP1, DCE-MRI, and TCR repertoire are potential biomarkers for therapeutic efficacy in patients with mRCC. Furthermore, the presence or absence of a history of antibiotics use was related to the prognosis of patients with mRCC undergoing IO therapy (Figure 1).
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-83/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-83/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-83/coif). KF has received a grant from Pfizer and honoraria from Pfizer, MSD, Ono, Bristol-Myers, and Takeda. MN has received honoraria from Pfizer, Merck Serono and Takeda. HU has received grants from Ono Pharm, and Takeda; contract fees from Ono Pharm, and Chugai Pharm; consulting fees from Ono Pharm; lecture fees from Takeda, MSD, Pfizer, Eisai, and BMS. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Bosma NA, Warkentin MT, Gan CL, et al. Efficacy and Safety of First-line Systemic Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis. Eur Urol Open Sci 2022;37:14-26. [Crossref] [PubMed]
- Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators. Lancet 1999;353:14-7. [Crossref] [PubMed]
- Pyrhönen S, Salminen E, Ruutu M, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol 1999;17:2859-67. [Crossref] [PubMed]
- Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:1280-9. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584-90. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449-56. [Crossref] [PubMed]
- Pérez-Valderrama B, Arranz Arija JA, Rodríguez Sánchez A, et al. Validation of the International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) prognostic model for first-line pazopanib in metastatic renal carcinoma: the Spanish Oncologic Genitourinary Group (SOGUG) SPAZO study. Ann Oncol 2016;27:706-11. [Crossref] [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722-31. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Pontes O, Oliveira-Pinto S, Baltazar F, et al. Renal cell carcinoma therapy: Current and new drug candidates. Drug Discov Today 2022;27:304-14. [Crossref] [PubMed]
- Fitzgerald KN, Lee CH. Personalizing First-Line Management of Metastatic Renal Cell Carcinoma: Leveraging Current and Novel Therapeutic Options. J Natl Compr Canc Netw 2022;20. [Crossref] [PubMed]
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380:1103-15. [Crossref] [PubMed]
- Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol 2020;31:1030-9. [Crossref] [PubMed]
- Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:1563-73. [Crossref] [PubMed]
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380:1116-27. [Crossref] [PubMed]
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378:1277-90. [Crossref] [PubMed]
- Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 2022;128:2085-97. [Crossref] [PubMed]
- Regan MM, Jegede OA, Mantia CM, et al. Treatment-free Survival after Immune Checkpoint Inhibitor Therapy versus Targeted Therapy for Advanced Renal Cell Carcinoma: 42-Month Results of the CheckMate 214 Trial. Clin Cancer Res 2021;27:6687-95. [Crossref] [PubMed]
- Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020;5:e001079. [Crossref] [PubMed]
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2021;384:829-41. [Crossref] [PubMed]
- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med 2021;384:1289-300. [Crossref] [PubMed]
- Atkins MB, Rini BI, Motzer RJ, et al. Patient-Reported Outcomes from the Phase III Randomized IMmotion151 Trial: Atezolizumab + Bevacizumab versus Sunitinib in Treatment-Naïve Metastatic Renal Cell Carcinoma. Clin Cancer Res 2020;26:2506-14. [Crossref] [PubMed]
- Motzer RJ, Powles T, Atkins MB, et al. Final Overall Survival and Molecular Analysis in IMmotion151, a Phase 3 Trial Comparing Atezolizumab Plus Bevacizumab vs Sunitinib in Patients With Previously Untreated Metastatic Renal Cell Carcinoma. JAMA Oncol 2022;8:275-80. [Crossref] [PubMed]
- Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007;104:3360-5. [Crossref] [PubMed]
- Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med 2012;366:2517-9. [Crossref] [PubMed]
- Tannir NM, Signoretti S, Choueiri TK, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab versus Sunitinib in First-line Treatment of Patients with Advanced Sarcomatoid Renal Cell Carcinoma. Clin Cancer Res 2021;27:78-86. [Crossref] [PubMed]
- Tykodi SS, Gordan LN, Alter RS, et al. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase 3b/4 CheckMate 920 trial. J Immunother Cancer 2022;10:e003844. [Crossref] [PubMed]
- Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 2015;212:139-48. [Crossref] [PubMed]
- Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998;92:4150-66. [Crossref] [PubMed]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Hakozaki K, Tanaka N, Takamatsu K, et al. Landscape of prognostic signatures and immunogenomics of the AXL/GAS6 axis in renal cell carcinoma. Br J Cancer 2021;125:1533-43. [Crossref] [PubMed]
- Grüllich C. Cabozantinib: a MET, RET, and VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res 2014;201:207-14. [Crossref] [PubMed]
- Lee CH, Voss MH, Carlo MI, et al. Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates. J Clin Oncol 2022;40:2333-41. [Crossref] [PubMed]
- Glen H. Lenvatinib therapy for the treatment of patients with advanced renal cell carcinoma. Future Oncol 2016;12:2195-204. [Crossref] [PubMed]
- Adachi Y, Kamiyama H, Ichikawa K, et al. Inhibition of FGFR Reactivates IFNγ Signaling in Tumor Cells to Enhance the Combined Antitumor Activity of Lenvatinib with Anti-PD-1 Antibodies. Cancer Res 2022;82:292-306. [Crossref] [PubMed]
- Peng Q, Qiu X, Zhang Z, et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun 2020;11:4835. [Crossref] [PubMed]
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020;383:1218-30. [Crossref] [PubMed]
- Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998;273:13313-6. [Crossref] [PubMed]
- Zhang T, George DJ. Immunotherapy and targeted-therapy combinations mark a new era of kidney cancer treatment. Nat Med 2021;27:586-8. [Crossref] [PubMed]
- Motzer RJ, Choueiri TK, McDermott DF, et al. Biomarker analysis from CheckMate 214: nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J Immunother Cancer 2022;10:e004316. [Crossref] [PubMed]
- Dalgliesh GL, Furge K, Greenman C, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 2010;463:360-3. [Crossref] [PubMed]
- Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 2012;44:751-9. [Crossref] [PubMed]
- Hsieh JJ, Chen D, Wang PI, et al. Genomic Biomarkers of a Randomized Trial Comparing First-line Everolimus and Sunitinib in Patients with Metastatic Renal Cell Carcinoma. Eur Urol 2017;71:405-14. [Crossref] [PubMed]
- Panda A, de Cubas AA, Stein M, et al. Endogenous retrovirus expression is associated with response to immune checkpoint blockade in clear cell renal cell carcinoma. JCI Insight 2018;3:e121522. [Crossref] [PubMed]
- Jung K, Fleischhacker M, Rabien A. Cell-free DNA in the blood as a solid tumor biomarker--a critical appraisal of the literature. Clin Chim Acta 2010;411:1611-24. [Crossref] [PubMed]
- Bronkhorst AJ, Ungerer V, Holdenrieder S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol Detect Quantif 2019;17:100087. [Crossref] [PubMed]
- Yamamoto Y, Uemura M, Fujita M, et al. Clinical significance of the mutational landscape and fragmentation of circulating tumor DNA in renal cell carcinoma. Cancer Sci 2019;110:617-28. [Crossref] [PubMed]
- Yamamoto Y, Uemura M, Nakano K, et al. Increased level and fragmentation of plasma circulating cell-free DNA are diagnostic and prognostic markers for renal cell carcinoma. Oncotarget 2018;9:20467-75. [Crossref] [PubMed]
- Motzer RJ, Banchereau R, Hamidi H, et al. Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer Cell 2020;38:803-817.e4. [Crossref] [PubMed]
- Yankeelov TE, Gore JC. Dynamic Contrast Enhanced Magnetic Resonance Imaging in Oncology: Theory, Data Acquisition, Analysis, and Examples. Curr Med Imaging Rev 2009;3:91-107. [Crossref] [PubMed]
- Morgan B, Thomas AL, Drevs J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol 2003;21:3955-64. [Crossref] [PubMed]
- Udayakumar D, Zhang Z, Xi Y, et al. Deciphering Intratumoral Molecular Heterogeneity in Clear Cell Renal Cell Carcinoma with a Radiogenomics Platform. Clin Cancer Res 2021;27:4794-806. [Crossref] [PubMed]
- Joshi K, Milighetti M, Chain BM. Application of T cell receptor (TCR) repertoire analysis for the advancement of cancer immunotherapy. Curr Opin Immunol 2022;74:1-8. [Crossref] [PubMed]
- Kato T, Kiyotani K, Tomiyama E, et al. Peripheral T cell receptor repertoire features predict durable responses to anti-PD-1 inhibitor monotherapy in advanced renal cell carcinoma. Oncoimmunology 2021;10:1862948. [Crossref] [PubMed]
- Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013;110:9066-71. [Crossref] [PubMed]
- Caesar R, Tremaroli V, Kovatcheva-Datchary P, et al. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab 2015;22:658-68. [Crossref] [PubMed]
- Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016;65:426-36. [Crossref] [PubMed]
- Yang X, Guo Y, Chen C, et al. Interaction between intestinal microbiota and tumour immunity in the tumour microenvironment. Immunology 2021;164:476-93. [Crossref] [PubMed]
- Fujita K, Matsushita M, Banno E, et al. Gut microbiome and prostate cancer. Int J Urol 2022;29:793-8. [Crossref] [PubMed]
- Matsushita M, Fujita K, Nonomura N. Influence of Diet and Nutrition on Prostate Cancer. Int J Mol Sci 2020;21:1447. [Crossref] [PubMed]
- Dzutsev A, Badger JH, Perez-Chanona E, et al. Microbes and Cancer. Annu Rev Immunol 2017;35:199-228. [Crossref] [PubMed]
- Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91-7. [Crossref] [PubMed]
- Derosa L, Routy B, Fidelle M, et al. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur Urol 2020;78:195-206. [Crossref] [PubMed]
- Kato T, Fujita K, Minami T, et al. Real-world efficacy and safety of nivolumab plus ipilimumab in untreated metastatic renal cell carcinoma, and the impact of previous nephrectomy on clinical outcome: Japanese multi-institutional retrospective study. Int J Clin Oncol 2022;27:1596-604. [Crossref] [PubMed]
Cite this article as: Sugimoto K, Fujita K, Minami T, Nozawa M, Yoshimura K, Esa A, Uemura H. A narrative review of precision medicine in metastatic renal cell carcinoma. AME Med J 2023;8:11.

