
Disseminated intravascular coagulation after cryoablation for metastatic pancreatic cancer: a case report
Highlight box
Key Findings
• Our case describes an episode of life-threatening disseminated intravascular coagulopathy (DIC) complicated by consumptive coagulopathy and severe bleeding after palliative cryoablation.
What is known and what is new?
• Cryoablation is emerging as a new and effective therapy for locally advanced solid tumors and symptom palliation in metastatic disease.
• Cryoablation utilizes argon or nitrogen gas to generate repeated freeze-thaw cycles in the tissue, reaching temperatures as low as −190 ℃.
• To our knowledge, the occurrence of DIC after cryoablation is rare.
What is the implication, and what should change now?
• DIC is an unusual but life-threatening complication of advanced pancreatic cancer.
• Patients undergoing cryoablation should be evaluated for subclinical procoagulant DIC and risk-assessed for the likelihood of thrombosis and bleeding before the procedure.
Introduction
Pancreatic cancer (PC) is the fourth most common cause of cancer-related death in the United States. Despite advancements in surgery and chemoradiation therapies, PC has a 5-year survival rate of only 11% in the United States (1). Pain is a major problem due to cancer invasion and compression of nerves locally and at distant sites due to metastasis. In recent years, tumor embolization and ablation modalities including microwave, radiofrequency, high-intensity focused ultrasound, ethanol and cryoablation have been used more commonly as palliative therapeutic options (2-11).
Cryoablation is minimally invasive, safe, and has been shown to increase survival and palliate pain for various solid malignancies (2-8). Cryoablation utilizes argon or nitrogen gas to generate repeated freeze-thaw cycles in the tissue, reaching temperatures as low as −190 ℃ (7). It can be performed via open surgical, laparoscopic, or percutaneous approaches.
Cellular death from cryoablation occurs by direct and indirect mechanisms (9,10). Direct injury to the cells is due to physical damage of cellular membranes by formation of intracellular ice crystals at temperatures below −20 ℃. Indirect injury is produced by local vascular stasis and thrombosis, and by activation of the immune response resulting from cellular necrosis with systemic release of cellular content, including DNA, RNA, heat shock proteins and damage-associated molecular patterns (DAMPs) that act as tumor-specific antigens. This cryoimmunologic effect, known as the abscopal effect, can affect cancer cells outside and distanced from the ablated tissue for antitumoral response (2,7,9). A few studies have reported safe and successful palliative use of cryoablation in patients with locally advanced and unresectable PC with no adverse reactions (4,5,11).
Patients with advanced or metastatic PC are prone to develop thromboembolic events and disseminated intravascular coagulation (DIC), with reported incidence between 17–57% (12). The pathogenesis of DIC in cancer involves the expression of tissue factor and thrombin by the malignant cells followed by platelet activation as part of angiogenesis and tumor metastasis (13). Treatment is generally supportive, and the condition is associated with a poor prognosis. To our knowledge, the occurrence of DIC after cryoablation is rare. Our objective is to describe a case of severe DIC after palliative bone tumor cryoablation in a patient with metastatic PC. We present this case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-23-13/rc).
Case description
A 47-year-old woman with no significant past medical history was diagnosed with PC and underwent an uncomplicated Whipple procedure with curative intent. A year later, she developed deep venous thrombosis and was treated with rivaroxaban 20 mg daily. Computed tomography (CT) revealed metastases to the abdominal lymph nodes, peritoneum, right femur, and surrounding soft tissue. She received systemic palliative chemotherapy with weekly gemcitabine [900 mg/m2 (1,500 mg)] and paclitaxel [100 mg/m2 (160 mg)] for 5 months and underwent right femur tumor excision, open reduction, and internal fixation, followed by radiation therapy. Shortly after she developed local femoral bone recurrence associated with disabling right leg pain, requiring high doses of opioids which affected her quality of life. She underwent an uneventful palliative cryoablation procedure of the proximal femoral lesion; however, the pain was not controlled, requiring a nerve block along with high doses of narcotics.
Two months later, the decision was made to proceed with a second palliative cryoablation of the two 5 cm large femur osseous metastases. Four days before the procedure, the rivaroxaban was stopped and the patient had a hemoglobin of 7.4 g/dL, platelets of 70,000/µL, and an international normalized ratio (INR) of 1.61 (Table 1). Her moderate anemia and thrombocytopenia were attributed to her progressive and metastatic PC and/or chemotherapy.
Table 1
| Laboratory Parameters | Pre-procedural (4 days earlier) |
Procedure day | At 24 h | At 48 h | At 72 h |
|---|---|---|---|---|---|
| Hgb (g/dL) | 7.4 | 5.1 | 5.5 | 5.2 | 6.6 |
| Platelets (/µL) | 70 | 28 | 45 | 48 | 44 |
| Fibrinogen (mg/dL) | NA | 36 | 87 | 137 | 164 |
| Haptoglobin (mg/dL) | NA | Undetectable | Undetectable | Undetectable | Undetectable |
| PT (s) | 19 | 25 | 19 | 18 | 18 |
| PTT (s) | 24.9 | 47 | 37 | 35 | 35 |
| INR | 1.61 | 2.29 | 1.59 | 1.54 | 1.42 |
| LDH (U/L) | NA | 1,823 | 1,564 | 1,565 | 2,967 |
| Bilirubin (mg/dL) | NA | 1.6 | 1.9 | 2.8 | 2.4 |
| ALT (U/L) | NA | 13 | 14 | 11 | 13 |
| AST (U/L) | NA | 45 | 40 | 43 | 83 |
| Absolute reticulocyte count, k/mcL | NA | 435 | NA | 284 | 303 |
| ESR | NA | 3 | NA | NA | NA |
Hgb, hemoglobin; PT, prothrombin time; PTT, partial thromboplastin time; INR, international normalized ratio; LDH, lactic dehydrogenase; ALT, alanine aminotransferase; AST, aspartate aminotransaminase; ESR, erythrocyte sedimentation rate; NA, not available.
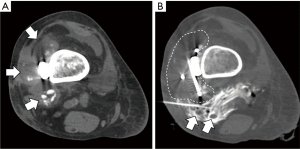
Figure 1A shows the large tumor recurrence in the distal femur, partially calcified, and infiltrating the soft tissues. Image-guided cryoablation was performed under CT and ultrasound guidance with the ablation applicator(s) advanced and positioned within the target(s) (Figure 1B). For each target lesion, the applicators were placed and repositioned as necessary to achieve the desired ablation zone. The ablation applicators were then removed, and sterile bandages were applied. Immediately post procedure, she developed slow but continuous blood oozing at the ablation site, which was difficult to control despite compression dressings, reinforcement sutures, and local thrombin powder. Her hemoglobin and platelets fell to 5.1 g/dL and 28,000 µL, respectively with an elevated INR of 2.29 and markedly low fibrinogen level of 36 mg/dL (range, 200–400 mg/dL), consistent with DIC (14) (Table 1). The patient was transferred to the intensive care unit (ICU) where she was noted to be hypotensive and tachycardic, with petechiae in both lower extremities. Peripheral blood smear revealed multiple schistocytes. CT angiogram of the right lower extremity did not show any bleeding vessel amenable to embolization. Laboratory studies over the next 3 days after the procedure are shown in Table 1. She was transfused multiple units of red blood cells, platelets, fresh frozen plasma, and cryoprecipitate.
Despite multiple daily transfusions, she continued to have pain and remained persistently thrombocytopenic and coagulopathic. The local wound bleeding was eventually controlled with extra enforced sutures, compression dressings, and local thrombin powder. Workup for sepsis, liver failure secondary to metastases, other microangiopathic syndromes, and antiphospholipid syndrome was negative. After discussion with the patient and her family, she chose to transition to comfort care measures and died during inpatient hospice.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was unable to be obtained from the patient for publication of this case report and accompanying images due to her being deceased prior to the compilation of this manuscript. Efforts were taken by all of the authors to not include any identifiable information regarding the patient within this manuscript.
Discussion
To our knowledge, the occurrence of severe DIC after percutaneous bone tumor cryoablation for metastatic PC has not been previously reported. Our case describes an episode of life-threatening DIC complicated by consumptive coagulopathy and severe bleeding after palliative cryoablation of two 5-cm large metastatic pancreatic tumors.
PC is among the most common solid malignancies associated with thromboembolic events, with a rate up to 36% in patients with advanced cancer. The spectrum of presentation varies widely from subacute and chronic abnormal coagulation tests, migratory superficial thrombophlebitis, deep venous thrombosis, marantic endocarditis, thrombotic microangiopathy, arterial thrombosis, and DIC (15).
The pathogenesis of cancer-associated DIC is multifactorial and complex (12). Procoagulant molecules like tissue factor positive microparticles are produced by tumor cells, released in circulation, and can initiate the coagulation cascade. Tumor cells ectopically expressing FVII and/or FX exhibit a prothrombotic effect by inducing platelet aggregation, activation and a dysregulatory effect on the fibrinolytic system.
Cryoablation is mostly used for palliation in metastatic PC. It is monitored intra-procedurally with CT or ultrasound, as there is clear visualization of the entire ice ball. Use of multiple cryoprobes allows for control of the size and shape of the ice ball to prevent the involvement of adjacent critical structures. Cellular injury can be influenced by variation in cooling and thawing rates, target, and time at target temperature (7). Limitations of cryoablation include incomplete killing of tumor cells around the margins of the ice ball and potential risk of freezing healthy tissues in cases where tumor is not readily visible by imaging (7).
Our patient suffered DIC with the repeated treatment of multiple and large tumors. We attribute the occurrence of DIC as a result of the necrotic malignant tissue that remains in situ after cryoablation that can stimulate immunologic responses and/or to the massive release of tissue factor in the circulation during cell death.
Since thrombocytopenia can be observed in patients with PC, patients undergoing cryoablation should be evaluated for subclinical procoagulant DIC and risk-assessed for the likelihood of thrombosis and bleeding before the procedure (16). Serial platelet count measurements should be obtained after the procedure particularly when a large volume tumor ablation is performed.
Conclusions
DIC is a life-threatening complication of advanced PC and can be exacerbated by large volume cryoablation. More studies are needed to understand the mechanism by which cryoablation enhances anti-tumor immunity and to risk stratify patients at risk of life threatening DIC.
Acknowledgments
Funding: This study was supported, in part, by the Core Grant (No. P30 CA008748) and the Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-23-13/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-13/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-13/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was unable to be obtained from the patient for publication of this case report and accompanying images due to her being deceased prior to the compilation of this manuscript. Efforts were taken by all of the authors to not include any identifiable information regarding the patient within this manuscript.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Survival rates for pancreatic cancer. Accessed December 6, 2022. Available online: https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html
- Aarts BM, Klompenhouwer EG, Rice SL, et al. Cryoablation and immunotherapy: an overview of evidence on its synergy. Insights Imaging 2019;10:53. [Crossref] [PubMed]
- Ravikumar TS, Kane R, Cady B, et al. A 5-year study of cryosurgery in the treatment of liver tumors. Arch Surg 1991;126:1520-3; discussion 1523-4. [Crossref] [PubMed]
- Patiutko IuI, Barkanov AI, Kholikov TK, et al. The combined treatment of locally disseminated pancreatic cancer using cryosurgery. Vopr Onkol 1991;37:695-700. [PubMed]
- Kovach SJ, Hendrickson RJ, Cappadona CR, et al. Cryoablation of unresectable pancreatic cancer. Surgery 2002;131:463-4. [Crossref] [PubMed]
- Korpan NN. Cryosurgery: ultrastructural changes in pancreas tissue after low temperature exposure. Technol Cancer Res Treat 2007;6:59-67. [Crossref] [PubMed]
- Kwak K, Yu B, Lewandowski RJ, et al. Recent progress in cryoablation cancer therapy and nanoparticles mediated cryoablation. Theranostics 2022;12:2175-204. [Crossref] [PubMed]
- Niu L, He L, Zhou L, et al. Percutaneous ultrasonography and computed tomography guided pancreatic cryoablation: feasibility and safety assessment. Cryobiology 2012;65:301-7. [Crossref] [PubMed]
- Baust JG, Snyder KK, Santucci KL, et al. Cryoablation: physical and molecular basis with putative immunological consequences. Int J Hyperthermia 2019;36:10-6. [Crossref] [PubMed]
- Cazzato RL, Garnon J, Ramamurthy N, et al. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol 2016;33:140. [Crossref] [PubMed]
- Wu Y, Gu Y, Zhang B, et al. Laparoscopic ultrasonography-guided cryoablation of locally advanced pancreatic cancer: a preliminary report. Jpn J Radiol 2022;40:86-93. [Crossref] [PubMed]
- Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol 2004;5:655-63. [Crossref] [PubMed]
- Levi M. Disseminated intravascular coagulation in cancer patients. Best Pract Res Clin Haematol 2009;22:129-36. [Crossref] [PubMed]
- Toh CH, Hoots WK. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost 2007;5:604-6. [Crossref] [PubMed]
- Campello E, Ilich A, Simioni P, et al. The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues. Br J Cancer 2019;121:359-71. [Crossref] [PubMed]
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 2009;145:24-33. [Crossref] [PubMed]
Cite this article as: Dulu A, Tayban Y, Delaleu J, Cornelis FH, Pastores SM. Disseminated intravascular coagulation after cryoablation for metastatic pancreatic cancer: a case report. AME Med J 2023;8:17.

