
Iatrogenic coronary artery injuries during non-coronary artery adult cardiac surgery
Introduction
Iatrogenic coronary artery injuries during non-coronary artery adult cardiac surgery are a constellation of serious complications associated with significant mortality and morbidity. These injuries are commonly the result of manipulation of the coronary artery with direct injury or distortion, embolization of particulate matter, inadvertent entrapment in sutures, or endothelial trauma due to energy transfer (1). The mechanisms of these injuries vary depending on the type of surgery being performed and despite their recognised occurrence these injuries are uncommon and underreported. The clinical presentations of these coronary injuries are dictated by the type of injury, the coronary artery involved, the location of the injury to the artery, and preoperative myocardial status (2). Timing of recognition of the injury dictates the therapeutic intervention. This review article provides an overview of iatrogenic coronary artery injuries during non-coronary artery adult cardiac surgery.
Injuries during aortic valve surgery
Coronary ostial stenosis following aortic valve replacement (AVR) is reported to occur in 1% to 5% of procedures involving the aortic valve (3). The stenosis can affect the left main coronary artery or the right coronary. However, majority of the reported cases involve the left main coronary artery (3,4). Acute coronary ostial injuries during AVR or aortic root replacement generally result from proximity to aortotomy or inclusion in aortotomy suture, coronary translocation, ostial laceration due to trauma from an aortic retractor, direct ostial cardioplegia cannula causing dissection, calcium debris embolization and partial or complete direct ostial occlusion by an oversized prosthesis (3) (Figure 1). Acute thromboembolic occlusion of a moderately stenosed coronary artery after protamine administration has also been reported as a potential mechanism of iatrogenic coronary injury after AVR (5).
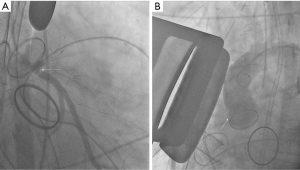
Delayed coronary ostial stenosis is attributed to the possibility of microtrauma and local fibrotic reaction secondary to the infusion pressure of the cardioplegic fluid and overdistension of the vessel by the tip of the cardioplegia delivery cannulas (6-8). An immunologic reaction to the porcine prosthesis is another potential mechanism resulting in delayed coronary ostial stenosis after AVR (9). Lastly, it is proposed that intimal thickening and fibrous hyperplasia secondary to turbulent flow around prosthetic valves can incite coronary ostial stenosis in the absence of coronary ostial manipulation (10).
Injuries during mitral valve surgery
Left circumflex coronary artery injury, with an incidence ranging from 0.5% to 1.8%, is a grave and sometimes lethal complication of surgical interventions on the mitral valve (1,11,12). The circumflex coronary artery lies within the atrioventricular groove, in close proximity to the posterior mitral valve annulus, adjacent to the anterolateral commissure making it vulnerable during mitral valve replacement or surgical annuloplasty (1).
A variety of mechanisms of iatrogenic circumflex artery injury have been described in literature. An anchoring suture of the prosthetic valve or annuloplasty ring completely encircles and obstructs the artery (13). Occasionally, a suture passes through and completely blocks the artery (13). Sometimes, dynamic or fixed blockage or distortion of the artery can be a consequence of a large quadrangular resection of the posterior mitral leaflet (14,15). Other reported injuries include laceration of the artery that results in contained bleeding (16), subintimal haematoma, coronary spasm, or total blockage secondary to clot formation (1). Direct injury of the artery by a suture is more commonly seen in left dominant circulation and in approximately 25% of the patients the artery lies within 3 mm of the mitral valve annulus (17). Hiltrop et al. (11) verify an enhanced risk of circumflex artery injury during mitral valve surgery in balanced or left-dominant coronary circulations. However, preoperative awareness of coronary anatomy does not prevent circumflex artery injury. An aberrant circumflex artery originating from the right coronary sinus has been recognised as a potential specific high-risk entity (11).
Injuries during tricuspid valve surgery
Due to the adjacency of the tricuspid annulus and the right coronary artery (RCA), injuries of the RCA have been reported secondary to surgical interventions on the tricuspid valve (18-22). Direct injury to the RCA is an infrequent but serious complication, probably underdiagnosed, which can produce substantial clinical effects (20). In a recently published study, RCA injury as defined by blockage needing emergency intervention/surgery was diagnosed in 12.3% of the patients who underwent post-operative coronary angiography (0.6% of all patients undergoing tricuspid valve surgery) (18). The same study reported a new RCA deformation without flow-impairment or vascular damage in 24.6% of the cases that was managed conservatively.
Coronary injury commonly develops between the right marginal artery and crux of the heart in affected patients. This site corresponds to the anteroposterior commissure and posterior leaflet annulus of the tricuspid valve along the right ventricular free wall. In more than 80% of patients, this is where the RCA is most closely related to the tricuspid annulus with a distance less than 5 mm (23). There are two possible mechanisms that can explain RCA injury during tricuspid valve surgery. The more likely one is direct obstruction with the suture. The other mechanism is deformation of the artery due to changes in annular shape. Annulus dilatation may distort the normal RCA course, and severely atherosclerotic, calcified and rigid arteries that are more prone to kinking experience plaque fracture and subsequent obstruction. Dilated annuli, which usually require more plication, probably have an increased risk of direct RCA injury during tricuspid annuloplasty, particularly when performing suture annuloplasty without a prosthetic ring (20).
Injuries during pulmonary valve surgery
The left main coronary artery is closely related to the pulmonary artery and its root. The first septal branch of the left anterior descending artery has an inconsistent trajectory and may occasionally be fairly sizeable. Harvesting of the pulmonary autograft can injure the first septal artery with potentially devastating consequences (24). Another mechanism of coronary artery injury is the potential distortion or compression of the coronary arteries during the reimplantation of the coronary buttons in the free-standing autograft (25).
Clinical presentation
The usual symptoms and clinical findings of iatrogenic coronary injury are dictated by the nature of injury. Acute injuries usually manifest intraoperatively or immediately postoperatively. Chronic injuries mostly present within the first 6 postprocedural months, but they may occur as late as 30 months postoperatively (26). Intraoperative features of acute injuries include bleeding, haemodynamic instability, refractory life threatening arrhythmias, ST changes, inability to wean from extracorporeal circulation, and acute right or left heart failure (19). The ECG changes, arrhythmias and acute haemodynamic instability can also occur due to air embolization. However, myocardial ischaemia secondary to coronary air embolization or spasm is reversible and usually settles within a short period if sufficient coronary perfusion is maintained (19).
Chronic injuries can present as acute coronary syndrome (27), unstable angina, exertional dyspnoea, congestive heart failure, arrhythmias, cardiogenic shock or sudden death (28).
Diagnosis
Intraoperative detection of iatrogenic coronary injuries requires a high index of suspicion. Early diagnosis is critical to mitigate the longer-term adverse effects of coronary stenosis or occlusion. Evaluation of 12-lead electrocardiogram enables detection of persistent ischaemic changes. Intraoperative transoesophageal echocardiography allows detailed assessment of ventricular function and can detect wall motion abnormalities (29).
Postoperative diagnosis is usually confirmed by conventional coronary angiography that should be performed emergently or urgently as dictated by the clinical condition of the patient.
Management
The principle of management of iatrogenic coronary injuries is to urgently restore myocardial blood flow to optimise haemodynamics and improve the prognosis (30). Revascularization of an occluded or compromised coronary artery can be achieved through percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery depending on the timing of identification of the injury. When injury is detected intraoperatively and prior to chest closure, coronary bypass grafting is the preferred approach (30,31). However, when the injury manifests after chest closure and transfer to intensive care unit, both PCI and CABG can be considered to restore myocardial blood flow (32). PCI is favoured in cases of coronary arterial kinking given the rapid nature of the procedure and avoidance of repeat sternotomy (33). However, when the vessel is obstructed completely by an encircling suture CABG is the preferred option (31). Hybrid operating room, when available, can be an ideal environment as both treatment modalities, PCI and CABG, can be offered as dictated by the haemodynamic status of the patient as well as the underlying mechanism of injury. It is important to mention that long-term results of either revascularisation strategy are unknown.
Conclusions
Iatrogenic injury to the coronary artery is a rare but potentially devastating complication of non-coronary artery adult cardiac surgery. Several mechanisms have been postulated to account for this iatrogenic complication. Swift diagnosis with a high index of suspicion is critical to prevent long-term adverse consequences. Management involves immediate restoration of coronary flow with PCI or CABG.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-106/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-106/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grande AM, Fiore A, Massetti M, et al. Iatrogenic circumflex coronary lesion in mitral valve surgery: case report and review of the literature. Tex Heart Inst J 2008;35:179-83. [PubMed]
- Pessa CJN, Gomes WJ, Catani R, et al. Anatomical relashionship between the posterior mitral valve annulus and the coronary arteries. Implication to operative treatment. Braz J Cardiovasc Surg 2004;19:372-7. [Crossref]
- Ziakas AG, Economou FI, Charokopos NA, et al. Coronary ostial stenosis after aortic valve replacement: successful treatment of 2 patients with drug-eluting stents. Tex Heart Inst J 2010;37:465-8. [PubMed]
- Pennington DG, Dincer B, Bashiti H, et al. Coronary artery stenosis following aortic valve replacement and intermittent intracoronary cardioplegia. Ann Thorac Surg 1982;33:576-84. [Crossref] [PubMed]
- Ackah JK, Dastidar AG, Angelini GD, et al. Acute coronary occlusion after surgical replacement of the aortic valve treated with emergency off-pump coronary artery bypass grafting. J Card Surg 2021;36:3877-80. [Crossref] [PubMed]
- Sethi GK, Scott SM, Takaro T. Iatrogenic coronary artery stenosis following aortic valve replacement. J Thorac Cardiovasc Surg 1979;77:760-7. [Crossref] [PubMed]
- Winkelmann BR, Ihnken K, Beyersdorf F, et al. Left main coronary artery stenosis after aortic valve replacement: genetic disposition for accelerated arteriosclerosis after injury of the intact human coronary artery? Coron Artery Dis 1993;4:659-67. [Crossref] [PubMed]
- Pande AK, Gosselin G. Iatrogenic left main coronary artery stenosis. J Invasive Cardiol 1995;7:183-7. [PubMed]
- Tsukiji M, Akasaka T, Wada N, et al. Bilateral coronary ostial stenosis after aortic valve replacement with freestyle stentless bioprosthesis: a case report. J Cardiol 2004;44:207-13. [PubMed]
- Rath S, Goor DA, Har-Zahav Y, et al. Coronary ostial stenosis after aortic valve replacement without coronary cannulation. Am J Cardiol 1988;61:1156-7. [Crossref] [PubMed]
- Hiltrop N, Bennett J, Desmet W. Circumflex coronary artery injury after mitral valve surgery: A report of four cases and comprehensive review of the literature. Catheter Cardiovasc Interv 2017;89:78-92. [Crossref] [PubMed]
- Aybek T, Risteski P, Miskovic A, et al. Seven years' experience with suture annuloplasty for mitral valve repair. J Thorac Cardiovasc Surg 2006;131:99-106. [Crossref] [PubMed]
- Virmani R, Chun PK, Parker J, et al. Suture obliteration of the circumflex coronary artery in three patients undergoing mitral valve operation. Role of left dominant or codominant coronary artery. J Thorac Cardiovasc Surg 1982;84:773-8. [Crossref] [PubMed]
- Meursing DF, Boonswang NA, Dobrilovic N, et al. Perioperative myocardial infarction secondary to dynamic circumflex coronary artery occlusion after mitral valve repair. Tex Heart Inst J 2006;33:85-7. [PubMed]
- Tavilla G, Pacini D. Damage to the circumflex coronary artery during mitral valve repair with sliding leaflet technique. Ann Thorac Surg 1998;66:2091-3. [Crossref] [PubMed]
- Mulpur AK, Kotidis KN, Nair UR. Partial circumflex artery injury during mitral valve replacement: late presentation. J Cardiovasc Surg (Torino) 2000;41:333-4. [PubMed]
- Paparella D, Squiccimarro E, Di Mauro M, et al. Acute iatrogenic complications after mitral valve repair. J Card Surg 2022;37:4088-93. [Crossref] [PubMed]
- Gerçek M, Omran H, Friedrichs KP, et al. Right coronary artery deformation and injury following tricuspid valve surgery. Front Cardiovasc Med 2022;9:987993. [Crossref] [PubMed]
- Urabe D, Ide M, Matsuoka M, et al. Iatrogenic right coronary artery occlusion during minimally invasive cardiac surgery-tricuspid annuloplasty-a case report. JA Clin Rep 2022;8:81. [Crossref] [PubMed]
- Díez-Villanueva P, Gutiérrez-Ibañes E, Cuerpo-Caballero GP, et al. Direct injury to right coronary artery in patients undergoing tricuspid annuloplasty. Ann Thorac Surg 2014;97:1300-5. [Crossref] [PubMed]
- Kainuma S, Yamamoto H, Yamada S, et al. Iatrogenic Injury of Right Coronary Artery During Tricuspid Annuloplasty. Circ J 2017;81:1956-8. [Crossref] [PubMed]
- Tsuchida K, Nishida K, Oda H, et al. Right coronary artery stenosis associated with tricuspid valve ring annuloplasty. Cardiovasc Interv Ther 2017;32:420-4. [Crossref] [PubMed]
- Al Aloul B, Sigurdsson G, Can I, et al. Proximity of right coronary artery to cavotricuspid isthmus as determined by computed tomography. Pacing Clin Electrophysiol 2010;33:1319-23. [Crossref] [PubMed]
- Sarioglu T, Erek E, Yalçinbas YK, et al. Pericardial collar modification for Ross procedure. Cardiovasc Surg 2003;11:229-30. [Crossref] [PubMed]
- Stulak JM, Burkhart HM, Sundt TM 3rd, et al. Spectrum and outcome of reoperations after the Ross procedure. Circulation 2010;122:1153-8. [Crossref] [PubMed]
- Bernelli C, Bezante GP, Brunelli C, et al. Iatrogenic left main coronary ostial stenosis after a Bentall procedure in an asymptomatic young man. Tex Heart Inst J 2012;39:393-7. [PubMed]
- Araszkiewicz A, Lesiak M, Urbanowicz T, et al. Acute coronary syndrome as a result of left main coronary artery stenosis after aortic valve replacement. A report of three cases. Postepy Kardiol Interwencyjnej 2013;9:150-4. [Crossref] [PubMed]
- Todaro MC, Ielasi A, Silvestro A, et al. An unusual case of cardiogenic shock late following surgical aortic valve replacement. J Cardiol Cases 2016;13:162-4. [Crossref] [PubMed]
- Ender J, Selbach M, Borger MA, et al. Echocardiographic identification of iatrogenic injury of the circumflex artery during minimally invasive mitral valve repair. Ann Thorac Surg 2010;89:1866-72. [Crossref] [PubMed]
- Webb JG, Lowe AM, Sanborn TA, et al. Percutaneous coronary intervention for cardiogenic shock in the SHOCK trial. J Am Coll Cardiol 2003;42:1380-6. [Crossref] [PubMed]
- Gaba P, Kaneko T, Kochar A, et al. Left circumflex artery injury following surgical mitral valve replacement: a case report. Eur Heart J Case Rep 2021;5:ytab464. [Crossref] [PubMed]
- Husain A, Alsanei A, Tahir M, et al. Left circumflex artery injury postmitral valve surgery, single center experience. J Saudi Heart Assoc 2019;31:94-9. [Crossref] [PubMed]
- Dello SA, Leus SJ, Tan MES, et al. Percutaneous coronary intervention of an iatrogenic occlusion of the circumflex coronary artery after mitral valve replacement. Eur Heart J Acute Cardiovasc Care 2020;9:NP1-2. [Crossref] [PubMed]
Cite this article as: Comanici M, Raja SG. Iatrogenic coronary artery injuries during non-coronary artery adult cardiac surgery. AME Med J 2023;8:12.

