
Second pleurectomy/decortication for a contralateral relapse of epithelial mesothelioma revealed by pneumothorax: a case report
Highlight box
Key findings
• Second pleurectomy/decortication can be a safe procedure for diagnostic and cytoreductive purpose in selected patients with contralateral mesothelioma relapse.
What is known and what is new?
• Second surgery for contralateral MPM relapse is rarely carried out.
• Few studies on second surgery are based on previous EPP and on solid pattern recurrence.
• This report describes a contralateral lung-sparing surgery performed for a recurrence unexpectedly occurred as a recalcitrant hydropneumothorax.
What is the implication, and what should change now?
• Further studies based on a second lung-sparing surgery for MPM contralateral relapse are needed.
• It should be clarified whether a surgical strategy based on lung-preservation can bring benefits to MPM relapsing patients, allowing greater tolerability towards further treatment, including a second surgery.
Introduction
Background
Malignant pleural mesothelioma (MPM) is a rare cancer related to professional and environmental asbestos exposure and is responsible of approximately 3,000 deaths per year in U.S. The incidence rate, with male predominance, varies from 1.8 to 2.5 cases per 100,000 and will decline in the coming years in those countries that have adopted regulatory laws on asbestos processing since the 1980s (1). The treatment for this high-mortality disease is founded on a multimodal approach that combines surgery, chemotherapy and radiotherapy.
Rationale and knowledge gap
The surgical-based upfront strategy, includes the more radical extrapleural pneumonectomy (EPP) and the lung-preserving pleurectomy/decortication (P/D), that could be extended to diaphragm [extended pleurectomy/decortication (EP/D)]. Since microscopically negative resection margins cannot be ensured, local and distant recurrences seem inevitable regardless of the type of technique (2-5). However, proponents of lung-sparing procedures argue that the benefits, due to the conservation of pulmonary function, allow relapsing patients to better tolerate further treatment, even if by surgery (6).
Objective
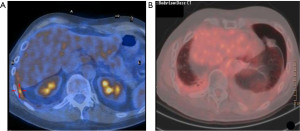
This report describes a case of a recalcitrant left pneumothorax that occurred as suspected MPM contralateral relapse, eleven years after a right EP/D (Figure 1). For this unusual presentation, a left P/D was carried out for diagnostic and treatment in order to obtain the maximal cytoreduction. We present this case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-23-63/rc).
Case presentation
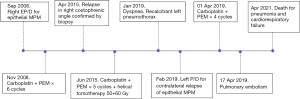
A 46-year-old Caucasian man with recurrent pleural effusion without asbestos exposure, in 2008 was diagnosed with a right-side epithelial MPM (Figure 2). In his clinical history never smoke, allergy to salicylates and his father deceased for MPM at the age of 70. An EP/D with the reconstruction of pericardium and hemi-diaphragm was performed to obtain the macroscopic complete removal (MCR). Epithelial mesothelioma staged pT1bN0 [7th Tumor, Node, Metastasis (TNM) staging edition] was confirmed at pathological report. Adjuvant systemic chemotherapy was administered according to the scheme pemetrexed (500 mg/m2) followed by carboplatin (75 mg/m2), on day 1 of a 21-day cycle for a total of 6 cycles. At the follow-up, the computed tomography scan (CT scan) by contrast was planned every 4 months. The follow-up was negative for relapse up to 2015. A local recurrence (Figure 1A), confirmed by needle biopsy, in the costophrenic angle at 7 years from EP/D was treated by 5 cycles of chemotherapy and helical tomotherapy (HT). Patient relapsed on the left side 11 years after EP/D, showing dyspnea for a recalcitrant hydropneumothorax (Figure 1B). Despite a correct management of the chest drain, pneumothorax re-appeared after any clamping test of the pleural tube. The 18F-fluorodeoxyglucose positron emission tomography scan (FDG-PET scan) was not exhaustive, showing a mild uptake on the left-side. Surgery was discussed in the multidisciplinary teamwork for diagnostic and, if appropriate, for maximal cytoreduction. The restrictive lung conditions, due to previous radiotherapy, were confirmed at the cardiopulmonary work-up in terms of forced expiratory volume in the first second (FEV1 1.77 L as 34%) and diffusing capacity of the lung for carbon monoxide divided by the alveolar volume (DLCO/VA 109%) and the arterial blood gas allowed the surgery (pO2 70 mmHg; SatO2 96%; pH 7.45; pCO2 44 mmHg). Through left uniportal videothoracoscopy, the access for the camera was prepared in 7th intercostal space on the posterior axillary line. At the exploration, the parietal and visceral pleural sheets appeared evenly thickened and biopsies were randomly performed in the cavity. The frozen section on parietal thickening was still doubtful but rather, the visceral pleura revealed a mesothelial proliferation consistent with a tumor relapse (Figure 3). After a conversion to vertical thoracotomy, a P/D was performed to aim the MCR. The parietal pleura was entirely detached and removed up to the costophrenic angle. The decortication was achieved completely on the lower lobe and partially on the upper lobe. The interlobar fissure was freed and carefully peeled with a blunt dissection, mostly at the surface between lingula and basal pyramid segments, where biopsies resulted positive for MPM. Final histopathology confirmed the recurrence of epithelial mesothelioma. A surveillance in intensive care unit (ICU) was scheduled for the first 24 hours after surgery. Postoperative course was uneventful and the discharge occurred in 6th day. Two months after the discharge, the patient experienced dyspnea and cardiac tachyarrhythmia. A CT pulmonary angiogram discovered a pulmonary embolism on the arterial branch for the right lower lobe that showed atelectasis downstream of arterial obstruction. The anticoagulant treatment with Rivaroxaban was administered until complete recovery of pulmonary blood flow. Further chemotherapy was administered with 4 cycles following the pemetrexed-carboplatin regimen mentioned above. After 12 months from P/D, the FDG-PET scan was negative and the six-minute walking test (6MWT) resulted within the normal limit, with the arterial blood gas being as follows: pCO2 43 mmHg, pO2 66 mmHg SatO2 96%. At 26 months from the left P/D, the patient deceased for a pneumonia not coronavirus disease 2019 (COVID-19)-related and concomitant cardiorespiratory failure. All procedures performed in this study were in accordance with the ethical standards of the Institutional Review Board of AULSS3 (No. 0090049 on 13-05-2022) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained due to the death of the patient and after all attempts were made, it was not possible to contact the patient’s familial members.
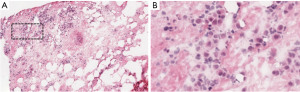
Discussion
Key findings
In our case, the initial local relapse appeared 7 years after P/D extended to diaphragm and pericardium in the ipsilateral hemithorax at the right costophrenic angle. The aspect of solid lesion (Figure 1A) confirmed by a biopsy as a relapse of epithelioid MPM, made the multidisciplinary board more inclined towards an approach with a helical intensity-modulated radiotherapy (IMRT). The suspicion of a contralateral recurrence arose 4 years later, when a recalcitrant left hydropneumothorax appeared (Figure 1B). After multimodal therapy, local and distant relapse of MPM represents the principal issue affecting survival for resected patients. The biological behavior that characterizes MPM as an extremely aggressive disease makes recurrence almost inevitable (2-6). The natural evolution of MPM differs from other solid tumors in the way it infiltrates adjacent tissues, resulting in a microscopic invasion that covers like a sheet all contiguous surfaces, leaving a boundary of normal tissue surrounded by disease (7). For this reason (8), the surgery-based treatment with both EPP and P/D can only be considered as R1. Indeed, the debate around the best surgical technique has always fluctuated between the more radical (EPP) and the more conservative procedure (P/D), in the belief that greater radicality could reflect a greater control over the margins of resection (9-11).
Strengths and limitations
Few studies have highlighted the role of second surgery for recurrences after resected MPM (Table 1) and most of them, based on previous EPP, did not recommend contralateral surgery. To date, in fact, there is a lack of standardized protocols of treatment in case of failure after multimodal approach in MPM resected patients and the choice to perform a redo-surgery is still limited. An important limitation of this paper is to describe an experience on a single case which prevents generalization of observed results. Moreover, this report is based on the outcomes of a long-survivor MPM patient for a period of 13 years (Figure 2) and many changes have occurred in MPM treatment strategy, in TNM grouping and in guidelines recommendations over the years. The uncommon presentation of MPM relapse as recalcitrant pneumothorax and the decision-making process in this rare condition that led to surgery as both diagnostic and treatment option represents the point of interest for those who have to manage the follow-up of this pathology.
Table 1
| Study, year | First surgery | Second surgery for relapse (n) | Type of surgical resections [n] |
|---|---|---|---|
| Politi, 2010, (12) | EPP | 8 | Chest wall [4]; retroperitoneal [2]; retroperitoneal + pectoral muscle [1]; contralateral upper lobe segmentectomy [1] |
| Burt, 2013, (13) | EPP | 47 | Chest wall [43]; chest wall + partial hepatectomy [2]; costophrenic + ipsilateral diaphragm [2] |
| Okamoto, 2013, (14) | EPP | 2 | Posterior mediastinum resection [1]; chest wall resection [1] |
| Kostron, 2016, (5) | EPP; P/D | 16 | Chest wall soft tissue [9]; extended chest wall [4]; contralateral partial pleurectomy + lung; wedge resection [3] |
| Nakamura, 2020, (3) | P/D | 3 | Soft tissue chest wall resection [3] |
| Bellini, 2021, (15) | EPP; P/D | 13 | Chest wall soft tissue [3]; ipsilateral pleura [2]; abdomen [1]; contralateral cheek [1]; contralateral lung wedge [2]; extended chest wall [1]; ipsilateral mastectomy [1]; peritonectomy + HIOC [1]; ipsilateral axillary adenopathy [1] |
EPP, extrapleural pneumonectomy; P/D, pleurectomy/decortication; HIOC, hyperthermic intraoperative chemotherapy.
Comparison with similar researches
After EPP the most frequent pattern of failure is considered as related to a distant spread involving contralateral hemithorax, peritoneum, abdomen, bone and brain with a longer disease-free survival (DFS) when compared to P/D, after which, the disease primarily relapses in the ipsilateral hemithorax. Bellini et al. reinforced this assumption (15) in a recent analysis on 250 resected MPM patients. They found that, the local relapse following P/D occurred earlier than those distant, being related to the greater amount of microscopic disease load left in place after surgery. Actually, whether the pattern of recurrence is linked to the type of surgery is still a matter of debate in literature. A research on a historical cohort of failure after trimodal therapy pivoted on EPP estimated that up to 75% of patients experienced recurrence with a median DFS of 13 months and a median survival of 15 months (2). The most frequent pattern of relapse was the ipsilateral hemithorax in 54% of patients. More recently, Kostron et al. have reached similar conclusions in their analysis (5), reporting a recurrence rate of 78% with a median DFS of 9 months after a multimodal approach based on EPP and induction chemotherapy. In the 106 relapsing cases, failure appeared at the ipsilateral hemithorax, including mediastinum, in 31% of patients. Comparing two groups of relapsing patients treated with EPP vs. P/D, Kai et al. found a similar pattern of recurrence (6), including local, distant and multiple sites, that was not dependent on the surgical technique (P=0.7). No difference was observed in the DFS among the groups (median DFS, 13 vs. 21 months; P=0.37). Zhou et al. conducted a comparative research (16) that matched the surgical outcomes of the two techniques with the propensity score, observing that both the distribution of recurrence and the DFS are overlapping between groups. These apparently contradictory results present in literature, seem to conclude that eliminating microscopic disease with a real R0 resection is theoretically impossible, since with both procedures the attempt to obtain surgical margins free from the disease is bound to fail in equal measure, even in case of more radical excision. Batirel et al. (17) indeed reported that following EPP, positive margins were found on 70% of specimens at final pathology report.
Explanations of findings
Recalcitrant hydropneumothorax at presentation is anecdotal for MPM patients and was described only in few case-series (18-20). The hydropneumothorax requires not only the presence of a chronic inflammatory process to produce the effusion, but also a stable patency of a broncho-pleural communication. Initially described in 1956 by Eisenstadt (21), the exact etiology of a persistent hydropneumothorax secondary to MPM is unclear and a different combination of mechanisms are implied to explain this rare phenomenon. Firstly, the rupture of the necrotic nodules of the tumor with the formation of broncho-pleural fistulas. Secondly, the hyperdistension of subpleural bubbles that break under the ball valve-like action of tumor nodules on the peripheral airways. Thirdly, the replacement and rupture of the pleural surface due to the direct spread of the tumor (18-20). For the case described in this report, a needle biopsy on the contralateral recurrence was not possible for the absence of an evident tumor burden at CT-scan and at FDG-PET. The unusual presentation of recurrence stood out for the aforementioned sheet-like invasion rather than a solid lesion development, for this reason, the decision towards surgery was taken for diagnostic purpose and to reach the maximal cytoreduction, since the disease was confirmed at frozen section.
Implications and actions needed
Recently a systematic review (22) analyzed the most common pattern of recurrence and the outcomes after second-line surgery-based treatment, providing an overview on the data available from six studies selected from literature (Table 1). Male gender, epithelioid histology, multimodal therapy (at least bimodal) and EPP as the upfront surgery were the predominant characteristics. Only in three studies patients underwent P/D as first surgical option. The most common pattern of relapse treated by redo-surgery with curative intent was a solid lesion at ipsilateral hemithorax and the chest wall resection was the intervention of choice. Second surgery for distant recurrence was carried out at contralateral hemithorax in six cases, performing three pulmonary resections and three partial pleurectomies. Of these six cases, only two patients underwent a previous P/D. The principal findings of the review concluded that second surgery is an acceptable option to treat MPM relapse in previously resected patients, even if the majority of cases included underwent mainly to EPP as first surgical treatment. To date the second surgery is the treatment of choice on 24.4% of MPM relapsing patients. The reported post-recurrence survival ranged from 14.5 to 23.5 months and benefits for survival derived from epithelioid histology and longer DFS. Local relapse and post-recurrence treatment have a favorable impact on the overall survival and no relations with the type of previous surgery have been found. According with these key-points, in our report, a second partial P/D was performed in presence of a diffuse non-solid infiltration of the contralateral pleura, reaching a post-recurrence survival of 26 months. This was possible because the patient remained fit for further treatments since his lung function had been preserved after the first lung-sparing surgery. For this reason, further studies, pivoted on P/D as the upfront surgery, are required to clarify the role of the second surgical treatment and to provide protocols for a possible second-line therapy for MPM recurrence.
Conclusions
In case of failure of multimodal treatment for epithelioid MPM, further studies are needed to confirm whether in selected cases after an upfront lung-sparing procedure, as P/D, a second lung-sparing surgery in case of contralateral recurrence can be safely performed. Moreover, due to the low postoperative complication rate related to a preservation of the lung function, this procedure can have a favorable impact also on the long-term outcomes since patients remain eligible for additional systemic therapy, improving the post-recurrence survival.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-23-63/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-63/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-63/coif). In 2022, SF received a grant from Astra Zeneca for his participation to an Advisory Board realized on behalf of Astra Zeneca: “advisory board chirurghi toracici triveneto”. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Institutional Review Board of AULSS3 (No. 0090049 on 13-05-2022) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained due to the death of the patient and after all attempts were made, it was not possible to contact the patient’s familial members.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carbone M, Adusumilli PS, Alexander HR Jr, et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin 2019;69:402-29. Erratum in: CA Cancer J Clin 2020;70:313-4. [Crossref] [PubMed]
- Baldini EH, Richards WG, Gill RR, et al. Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2015;149:1374-81. [Crossref] [PubMed]
- Nakamura A, Takuwa T, Hashimoto M, et al. Clinical Outcomes With Recurrence After Pleurectomy/Decortication for Malignant Pleural Mesothelioma. Ann Thorac Surg 2020;109:1537-43. [Crossref] [PubMed]
- Breda C, Furia S, Lucchini G, et al. Long-term outcomes after lung-sparing surgery for epithelial mesothelioma. J Thorac Dis 2021;13:6283-93. [Crossref] [PubMed]
- Kostron A, Friess M, Crameri O, et al. Relapse pattern and second-line treatment following multimodality treatment for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;49:1516-23. [Crossref] [PubMed]
- Kai Y, Tsutani Y, Tsubokawa N, et al. Prolonged post-recurrence survival following pleurectomy/decortication for malignant pleural mesothelioma. Oncol Lett 2019;17:3607-14. [Crossref] [PubMed]
- Hung YP, Chirieac LR. Pathology of Malignant Pleural Mesothelioma. Thorac Surg Clin 2020;30:367-82. [Crossref] [PubMed]
- Flores RM. Pleurectomy decortication for mesothelioma: The procedure of choice when possible. J Thorac Cardiovasc Surg 2016;151:310-2. [Crossref] [PubMed]
- Wolf AS, Daniel J, Sugarbaker DJ. Surgical techniques for multimodality treatment of malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:132-48. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Danuzzo F, Maiorca S, Bonitta G, et al. Systematic Review and Meta-Analysis of Pleurectomy/Decortication versus Extrapleural Pneumonectomy in the Treatment of Malignant Pleural Mesothelioma. J Clin Med 2022;11:5544. [Crossref] [PubMed]
- Politi L, Borzellino G. Second surgery for recurrence of malignant pleural mesothelioma after extrapleural pneumonectomy. Ann Thorac Surg 2010;89:207-10. [Crossref] [PubMed]
- Burt BM, Ali SO, DaSilva MC, et al. Clinical indications and results after chest wall resection for recurrent mesothelioma. J Thorac Cardiovasc Surg 2013;146:1373-9; discussion 1379-80. [Crossref] [PubMed]
- Okamoto T, Yano T, Haro A, et al. Treatment for recurrence after extrapleural pneumonectomy for malignant pleural mesothelioma: A single institution experience. Thorac Cancer 2013;4:66-70. [Crossref] [PubMed]
- Bellini A, Dell'Amore A, Terzi S, et al. Relapse Patterns and Tailored Treatment Strategies for Malignant Pleural Mesothelioma Recurrence after Multimodality Therapy. J Clin Med 2021;10:1134. [Crossref] [PubMed]
- Zhou N, Rice DC, Tsao AS, et al. Extrapleural Pneumonectomy Versus Pleurectomy/Decortication for Malignant Pleural Mesothelioma. Ann Thorac Surg 2022;113:200-8. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol 2008;3:499-504. [Crossref] [PubMed]
- Mitsui A, Saji H, Shimmyo T, et al. Malignant pleural mesothelioma presenting as a spontaneous pneumothorax. Respirol Case Rep 2015;3:9-12. [Crossref] [PubMed]
- Sattar N, Durrance R, Khan A, et al. Malignant mesothelioma presenting as recurrent hydro-pneumothorax: An atypical case presentation and literature review. Respir Med Case Rep 2018;23:152-5. [Crossref] [PubMed]
- Makidono A, Matsusako M, Oikado K, et al. Malignant pleural mesothelioma detected by spontaneous pneumothorax. Jpn J Radiol 2010;28:547-51. [Crossref] [PubMed]
- Eisenstadt HB. Malignant mesothelioma of the pleura. Dis Chest 1956;30:549-56. [Crossref] [PubMed]
- Bellini A, Mazzarra S, Sterrantino S, et al. Second Surgery for Recurrent Malignant Pleural Mesothelioma after Multimodality Treatment: A Systematic Review. J Clin Med 2022;11:3340. [Crossref] [PubMed]
Cite this article as: Furia S, Natale G, Cavallin R, Ferronato A, Lo Giudice F, Breda C. Second pleurectomy/decortication for a contralateral relapse of epithelial mesothelioma revealed by pneumothorax: a case report. AME Med J 2023;8:38.

