
Nephron-sparing surgery for small renal masses: a narrative review
Introduction
Background
Over the last few years, the incidence of renal cell carcinoma (RCC) has increased greatly. One of the explanations for this phenomenon is the widespread use of imaging exams which leads to an increase in incidentally detected small renal mass (SRM) (1). Greater knowledge about the biology of renal tumors has allowed a more refined surgical approach, limiting the potential morbidity of long-term chronic kidney disease (CKD) and optimizing the preservation of renal parenchymal function (2).
In the past, radical nephrectomy (RN) with excision of Gerota’s fascia, followed by hilar lymphadenectomy and resection of the ipsilateral adrenal gland was the gold standard in oncologic resections for kidney cancer. However, the high postoperative morbidity rate limited its use until 1950, when Vermooten suggested that capsular and peripheral renal tumors should be resected, leaving a healthy parenchymal margin around the tumor (3).
Nephron-sparing surgery (NSS), therefore, consists of complete excision of the tumor, while preserving as much of the normal functional parenchyma of the affected kidney as possible (4), making it the standard to treat SRM. The European Association of Urology (EAU) guidelines recommends routine use of NSS for cT1 tumors (5).
The classic and imperative indications for partial nephrectomy (PN) are: localized renal tumors whose resection would lead the patient to an immediate anephric state and requiring hemodialysis (for instance: previous contralateral nephrectomy, renal agenesis and irreversible impairment of the contralateral renal function due to a previous dysfunction—tumor in a single functioning kidney—and bilateral synchronous tumors). Relative indications of NSS are potentially threatening conditions of renal function over the years, such as vesicoureteral reflux, recurrent or chronic pyelonephritis, renal artery stenosis, arterial hypertension, diabetes mellitus (DM), glomerulopathies and nephrosclerosis (6). Von Hippel-Lindau (VHL) syndrome patients and others RCC Hereditary syndromes, who have genetic alterations for clear RCC, may be eligible for partial surgery, given the potential risk for developing contralateral tumors especially in younger ones (2).
Success rate of NSS is high, and both morbidity and mortality rates are considered low (1% to 2%) (2). In RCC, cancer-free survival outcomes are similar to those obtained with RN for early-stage and localized disease. The incidence of recurrence is 2% to 4% (4). In tumors equal to or smaller than 4 cm, recurrence is even lower—between 0% and 3% (2). The recent multi-institutional comparative analysis of complex renal masses (CRMs) entitled ‘Partial or radical nephrectomy for complex renal mass: a comparative analysis of oncological outcomes and complications from the ROSULA (Robotic Surgery for Large Renal Mass) Collaborative Group’ have compared outcomes of 926 patients submitted to robotic-assisted partial nephrectomy (RAPN) and minimally invasive radical nephrectomy (MIS-RN) for complex renal masses (CRM). Cerrato et al. found RAPN in CRM is not associated with increased risk of complications or worsened oncological outcomes when compared to MIS-RN and may be preferred when clinically indicated (7).
Standard partial nephrectomy (SPN) surgical technique safety margin was raised in 1950 by Vermooten (3) and involves excising a 1 cm margin of normal parenchyma around the tumor. However, one centimeter of margin around the entire tumor results in a significant amount of healthy renal parenchyma, and thus leading to functional renal decline, in part related to the devascularization that can occur during the procedure (8,9).
Over time, data from some studies challenged this arbitrary 1 cm margin, they suggested that the thickness of parenchyma surrounding the tumor does not influence long-term progression-free survival in patients (10,11). A tendency to save as much renal parenchyma as possible became increasingly evident and NSS techniques have gained popularity.
Most studies show that the new-baseline glomerular filtration rate (GFR) after PN is an important predictor of long-term survival, especially for those with CKD (12,13).
It has already been shown that the amount of residual parenchyma is more important than ischemia time to predict postoperative renal function in patients undergoing PN (14). Minervini et al. proposed a standardization of NSS techniques, and divided into three main categories: simple enucleation (SE), enucleoresection (ER), and wedge resection (WR) (15).
Rationale, knowledge gap and objective
Several ways of performing NSS are described in the literature. However, the standardization of these approaches may not be as clear as one thinks (6). With this narrative review, we intend to show the different ways of performing PN, demonstrating the advantages and disadvantages of each method and the evolution of techniques over time. Due to the large number of PN techniques and considering the advances in minimally invasive techniques for preserving the renal parenchyma and positive oncological outcome, the authors bring this narrative review in order to summarize what is most consolidated about surgical technique and refinement about access to the renal parenchyma. We present this article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-105/rc).
Methods
We searched PubMed for English-language sources using the following keywords: partial nephrectomy, nephron sparing surgery, enucleation, simple enucleation, enucleoresection, wedge resection, polar nephrectomy and heminephrectomy. Articles on a particular surgical technique, case reports, systematic and non-systematic reviews and international urology guidelines were selected, as well as renowned books [1950–2022]. Due to the historical nature of the subject, we did not exclude articles published in last decades from the review. We searched the bibliographies of the retrieved articles written by experts in renal cancer area. We discuss some points and guidelines of relevant professional associations. Opinions expressed in this review are also based on personal experience. Table 1 summarizes the methodology.
Table 1
| Items | Specification |
|---|---|
| Date of search | December 14th, 2022 |
| Databases and other sources searched | PubMed, Campbell Urology |
| Search terms used | Nephrectomy, partial nephrectomy, nephron sparing surgery, enucleation, enucleoresection, wedge resection, heminephrectomy, polar nephrectomy |
| Timeframe | 1890–2022 |
| Inclusion and exclusion criteria | Inclusion criteria: articles written in English. Exclusion criteria: articles without full/open access were excluded |
| Selection process | All authors were responsible for data collection. Text was divided into sections and each author was designed to write a section. All authors revised final text |
PN modalities
Enucleation
Some studies have shown that enucleation can enhance renal parenchyma preservation when compared to SPN (16).
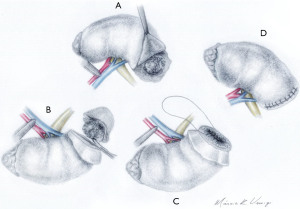
SE means removing the tumor using blunt dissection following the plane between the peritumoral pseudocapsule and normal parenchyma, without removing visible healthy tissue (Figure 1). This dissection plan is a landmark. When taken as a reference, it proved to be oncologically safe and with less bleeding (17). SE is feasible because of the pseudocapsule present in most tumors. Furthermore, due to tissue differences between the tumor and healthy parenchyma interface (18).
Dong et al. performed a study to compare functional outcomes between tumor enucleation and SPN. They showed that using enucleation, it was possible to preserve a greater amount of renal parenchyma. This impact was also reflected in the GFR. In addition, CKD was much less common after SE (19).
When discussing possible advantages of SE, we should mention less need for suturing the capsule. Due to the possibility of tumor dissection without sectioning adjacent vessels, the chance of bleeding during the excision phase is lower. In this way, the need to close the capsule in some cases can be dispensed (20). Probably due to the devascularization caused by suturing renal parenchyma and capsule, renorrhaphy is associated with a decrease in functional recovery after PN. One study showed that in groups in which capsular suture was not performed, there was less parenchymal loss (16% vs. 4%; P<0.001) and GFR loss (9% vs. 2%; P=0.03) (9).
It has already been shown that most of the lost renal parenchyma is due to the devascularization that occurs during the reconstruction phase in PN (21).
Enucleation of renal masses can be performed avoiding clamping of hilar renal vessels, especially for cortical tumors (22). This is very useful for patients who are known to be at higher risk for kidney failure.
At the beginning of the diffusion of the enucleation technique, it was believed that performing it through minimally invasive surgery would be more challenging, due to the loss of tactile sensation. However, several authors have been applying this technique safely and with good results. Dong et al. demonstrated in their work that laparoscopic or robot-assisted enucleation is safe from an oncological point of view (23).
There is concern regarding the invasion of the pseudocapsule. Minervini et al. showed that this occurs in 33% of cases. However, in all cases of their study, the margins were negative (18). Ficarra and colleagues hypothesized that chronic inflammation in the healthy tissue around the tumor could cause this tissue to remain around the surgical piece during blunt dissection (24).
Carini et al. (25) reported long-term follow-up data on a series of 232 patients undergoing enucleation for RCCs less than 4 cm with a median follow-up of 76 months. They demonstrated cancer-specific survival at 5 and 10 years of 96.7% and 94.7%, respectively, with progression-free survival at 5 and 10 years of 96% and 94%, respectively. In addition, there were no positive surgical margins (PSMs) and no local recurrences at the level of the enucleation bed (25).
Performing frozen biopsies of the base of the tumor has already been reported. However, they realized that there are some pitfalls. The main findings were atypical cells and renal tubules. Normal parenchymal components such as tubules and glomeruli can be misinterpreted as neoplasia. For this reason, the usefulness of these intraoperative biopsies remains questionable (26).
Another point of discussion is the ablation of the enucleation bed. However, there is data that suggests that enucleation without ablation may have similar oncologic outcomes (27).
This approach is especially useful for patients who renal function is a major concern. Besides that, it can be a useful strategy when tumors are in a hilar position, when excise a margin of normal parenchyma is challenging. Using this strategy, hilar vessels clamping can be avoided, decreasing the need for warm ischemia.
Between the methods of NSS, enucleation is the technique with the greatest potential to preserve more normal parenchyma and often avoids renal vessels clamping. Concerns about pseudocapsule tumor invasion, did not traduced in different progression free rates. It can be performed when the mass is near the hilum, a situation that excising a margin of normal parenchyma is not possible. SE is a conservative surgery in the functional aspect and respects the oncological principles.
Enucleoresection
Also referred as “excavation” (28), the ER technique consists on a resection with minimal margin (≥1 mm) but without contacting the pseudocapsule, maintaining only the contour of the renal tumor (18).
Adamakis et al., based on older studies, stated that SE presented a higher risk of compromised surgical margins subsequently to invasion of the tumor pseudocapsule, especially in bulking neoplasias (29), and therefore encourages the practice of ER over SE.
While some authors argue that this minimum margin tends to respect more the oncological principles, maintaining the benefit of preserving the renal parenchyma compared to SPN, others tend to question whether this benefit really exists in terms of PSMs, as demonstrated by Di Maida et al. where in their multivariable analysis, ER was indicated as an independent predictor of PSM on concluding anatomopathological report (HR: 2.68; 95% CI: 1.25–7.63; P=0.04) (30).
Because of the poor compile for standardization of surgical approach for PN on SRM, no definitive conclusions can be drawn to date on the oncological advantage of a specific technique over another (31).
WR
Having a self-explanatory name, WR advocates a triangle-shaped slice of the kidney maintaining a margin of healthy tissue and the tumor contour should not be visible (32). Eighteen years after the first nephrectomy by Gustav Simon, surgeon Vincenz Czerny performed the first PN in 1887 (33). Since then, the idea of renal preservation has been widespread, WR was one of the first techniques adopted after the standardization of SPN.
On early series, as mentioned by McDougall et al., WS was indicated only for tumors up to 2 cm, due to extensive conversion rate and more complication for patients with larger tumors (34).
Clearly with the evolution of techniques, new hemostatic agents and advanced minimally invasive platforms this is no longer an issue (35).
Heminephrectomy (or polar nephrectomy)
The definition of heminephrectomy is the excision of 30% or more of the renal parenchyma (31). In any PN technique, the surgical steps remain the same: vascular control and control of renal ischemia time, complete tumor excision with negative margins, strict closure of the collecting system, well done hemostasis and closure of the renal parenchyma (32). Figure 1 describes and illustrates in details the technique.
Laparoscopic PN was introduced by Clayman et al. in 1992, initially for benign diseases and now for all types of renal diseases, including RN and PN for RCC (36). The surgical principles remain the same as for open surgery: safe vascular control, limited renal ischemia and strict hemostasis (2). Contraindications are renal vein or inferior vena cava thrombi, larger tumor size and local tumor invasion of adjacent structures and lymph nodes. Centrally located tumors may have indication for RN in challenging cases (under surgeon’s discretion), not being considered an absolute contraindication (2).
Partial polar nephrectomy (heminephrectomy) consists of polar segmental nephrectomy with pre-clamping of the appropriate renal artery (32). The first step of the technique consists of dividing the blood supply from that segment to the renal pole (32). The renal capsule is then dissected and the renal parenchyma below is excised with the nourishing renal pedicle clamped, obstructing arterial blood. After the removal, the collecting system is closed with absorbable suture to prevent the formation of a urinary fistula. The components of the collecting system can be identified centrally while the parenchyma is sutured (3). The vascular clamp is removed and local hemostasis is observed. Regardless of the type of approach (open, laparoscopic, robotic), one should minimize warm ischemia time to less than 30 minutes (maximum 1 hour), as this period has been proven not to impact long-term renal function (2). Hypothermia can be considered for more complex cases or when ischemia time is known to be long (5). Intravenous heparin is not routinously recommended.
The renal capsule then serves as the second suture plane, closing the kidney (32). A hemostatic agent (or Gerota’s fat) can be used over the second suture plane (3). Attention should be paid to the use of thermal energy close to the suture planes, to avoid thermal destruction to the collecting system, remembering that late necrosis can be a cause of urinary fistula. In some cases, it may be necessary to place a drain in the suture bed to monitor possible fistula (2,32).
Occasional benign tumors after surgery and conservative therapies for SRMs: option to PN
Due to the increase in the diagnosis of SRM, which are incidental findings in routine examinations, the rate of benign tumors diagnosed after PN surgery has also increased and is estimated at 27% after the anatomopathological examination of the surgical specimen (37). This rate leads to a discussion about the risks and benefits of surgery, even partial surgery, for SRM. The Italian study by Baio et al. published in February 2023 in the journal Diseases studied the incidence of benign tumors after laparoscopic PN (37). Of 195 patients operated with laparoscopic PN, 30 tumors were diagnosed as benign, with a mean size of 3 cm (37). The tumors varied between oncocytomas, angiomyolipomas and cysts. Such findings should serve as a wake-up call for patients to be aware that there is a chance that surgery findings are benign, despite the risk (37).
SRM also allow follow-up through active surveillance of kidney lesions smaller than 2–3 cm due to the higher incidence of benign pathology. Such lesions can be monitored with computed tomography (CT) or serial magnetic resonance (MR) and have the size of the lesion followed (37). In case of evidence of growth or high growth velocity, these tumors are usually operated. Active surveillance may obviate the need for more unnecessary surgical treatments, with its consequent morbidity (37).
Another option to PN are minimally invasive interventional radiology techniques, such as radioablation and cryoablation (37). Special cases, such as in older patients, with high surgical risk, or bilateral synchronous tumors, or difficult surgical access for renal preservation, or even in chronic renal patients who need as much renal parenchyma as possible, may be candidates for these therapies, as long as the patient’s follow-up can be rigorous, preferably in oncology centers, as there is a greater risk of local tumor recurrence compared to partial techniques (37).
Discussion
PN, whenever available, remains the gold standard for the treatment of renal tumors. The surgeon’s experience will directly impact the choice of the best technique, in addition to the location of the tumor in the renal parenchyma. The amount of remaining renal parenchyma is more important than the ischemic time for future renal function. Margins smaller than 1 cm, as long as they do not violate the tumor pseudocapsule, may be safe from an oncological point of view. This study has literary limitations, as it was carried out only in the PubMed database and only articles in English were selected. More future studies of prosthetic design are needed to monitor the evolution of renal function in partially nephrectomized patients, as well as the oncological outcome and tumor recurrence in minimally invasive techniques. Figure 2 describes and illustrates all techniques.
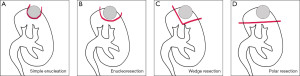
Summary
Research protocols could be implemented in surgical and oncology centers with a focus on the segment of these patients in the long-term, especially in complex renal tumors, close to the collecting system or vessels of the renal hilum, in order to study tumor margins and the outcome of renal function of the parenchyma.
Conclusions
NSS is the standard of care for SRMs. More aggressive techniques in terms of parenchymal preservation such as SE, ER or even WR has comparable long-term progression free and cancer specific survival to SPN. Concern about pseudocapsule tumor invasion, did not traduced in different progression free rates. The resection technique for PN (SE vs. ER vs. WR vs. SPN) is chosen by the surgical team after analysis of the entire tumor, including growth and degree of remaining healthy renal parenchyma, as long as negative blood margins are maintained.
Acknowledgments
Authors acknowledged institutions AC Camargo Cancer Center and Federal University of São Paulo and AME Medical Journal (AMJ) invitation.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Renal Cell Carcinoma”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-105/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-105/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-105/coif). The series “Renal Cell Carcinoma” was commissioned by the editorial office without any funding or sponsorship. SCZ served as an unpaid Guest Editor of the series. He also serves as an unpaid editorial board member of the AME Medical Journal from June 2022 to May 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Palumbo C, Pecoraro A, Knipper S, et al. Contemporary Age-adjusted Incidence and Mortality Rates of Renal Cell Carcinoma: Analysis According to Gender, Race, Stage, Grade, and Histology. Eur Urol Focus 2021;7:644-52. [Crossref] [PubMed]
- Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol 2001;166:6-18. [Crossref] [PubMed]
- Vermooten V. Indications for conservative surgery in certain renal tumors: a study based on the growth pattern of the cell carcinoma. J Urol 1950;64:200-8. [Crossref] [PubMed]
- Campbell SC, Novick AC, Streem SB, et al. Complications of nephron sparing surgery for renal tumors. J Urol 1994;151:1177-80. [Crossref] [PubMed]
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol 2022;82:399-410. [Crossref] [PubMed]
- Ghavamian R. Atlas of Laparoscopic and Robotic Urologic Oncological Surgery. New Delhi: Jaypee; 2013.
- Cerrato C, Patel D, Autorino R, et al. Partial or radical nephrectomy for complex renal mass: a comparative analysis of oncological outcomes and complications from the ROSULA (Robotic Surgery for Large Renal Mass) Collaborative Group. World J Urol 2023;41:747-55. [Crossref] [PubMed]
- Mir MC, Campbell RA, Sharma N, et al. Parenchymal volume preservation and ischemia during partial nephrectomy: functional and volumetric analysis. Urology 2013;82:263-8. [Crossref] [PubMed]
- Bahler CD, Dube HT, Flynn KJ, et al. Feasibility of omitting cortical renorrhaphy during robot-assisted partial nephrectomy: a matched analysis. J Endourol 2015;29:548-55. [Crossref] [PubMed]
- Lee JH, Chie EK, Kim K, et al. The influence of the treatment response on the impact of resection margin status after preoperative chemoradiotherapy in locally advanced rectal cancer. BMC Cancer 2013;13:576. [Crossref] [PubMed]
- Piper NY, Bishoff JT, Magee C, et al. Is a 1-CM margin necessary during nephron-sparing surgery for renal cell carcinoma? Urology 2001;58:849-52. [Crossref] [PubMed]
- Lane BR, Demirjian S, Derweesh IH, et al. Survival and Functional Stability in Chronic Kidney Disease Due to Surgical Removal of Nephrons: Importance of the New Baseline Glomerular Filtration Rate. Eur Urol 2015;68:996-1003. [Crossref] [PubMed]
- Demirjian S, Lane BR, Derweesh IH, et al. Chronic kidney disease due to surgical removal of nephrons: relative rates of progression and survival. J Urol 2014;192:1057-62. [Crossref] [PubMed]
- Ginzburg S, Uzzo R, Walton J, et al. Residual Parenchymal Volume, Not Warm Ischemia Time, Predicts Ultimate Renal Functional Outcomes in Patients Undergoing Partial Nephrectomy. Urology 2015;86:300-5. [Crossref] [PubMed]
- Minervini A, Carini M, Uzzo RG, et al. Standardized reporting of resection technique during nephron-sparing surgery: the surface-intermediate-base margin score. Eur Urol 2014;66:803-5. [Crossref] [PubMed]
- Blackwell RH, Li B, Kozel Z, et al. Functional Implications of Renal Tumor Enucleation Relative to Standard Partial Nephrectomy. Urology 2017;99:162-8. [Crossref] [PubMed]
- Minervini A, Carini M. Tumor Enucleation Is Appropriate During Partial Nephrectomy. Eur Urol Focus 2019;5:923-4. [Crossref] [PubMed]
- Minervini A, di Cristofano C, Lapini A, et al. Histopathologic analysis of peritumoral pseudocapsule and surgical margin status after tumor enucleation for renal cell carcinoma. Eur Urol 2009;55:1410-8. [Crossref] [PubMed]
- Dong W, Gupta GN, Blackwell RH, et al. Functional Comparison of Renal Tumor Enucleation Versus Standard Partial Nephrectomy. Eur Urol Focus 2017;3:437-43. [Crossref] [PubMed]
- Gupta GN, Boris RS, Campbell SC, et al. Tumor Enucleation for Sporadic Localized Kidney Cancer: Pro and Con. J Urol 2015;194:623-5. [Crossref] [PubMed]
- Dong W, Wu J, Suk-Ouichai C, et al. Devascularized Parenchymal Mass Associated with Partial Nephrectomy: Predictive Factors and Impact on Functional Recovery. J Urol 2017;198:787-94. [Crossref] [PubMed]
- Kutikov A, Vanarsdalen KN, Gershman B, et al. Enucleation of renal cell carcinoma with ablation of the tumour base. BJU Int 2008;102:688-91. [Crossref] [PubMed]
- Dong W, Chen X, Huang M, et al. Long-Term Oncologic Outcomes After Laparoscopic and Robotic Tumor Enucleation for Renal Cell Carcinoma. Front Oncol 2020;10:595457. [Crossref] [PubMed]
- Ficarra V, Galfano A, Cavalleri S. Is simple enucleation a minimal partial nephrectomy responding to the EAU guidelines' recommendations? Eur Urol 2009;55:1315-8. [Crossref] [PubMed]
- Carini M, Minervini A, Masieri L, et al. Simple enucleation for the treatment of PT1a renal cell carcinoma: our 20-year experience. Eur Urol 2006;50:1263-8; discussion 1269-71. [Crossref] [PubMed]
- Laryngakis NA, Van Arsdalen KN, Guzzo TJ, et al. Tumor enucleation: a safe treatment alternative for renal cell carcinoma. Expert Rev Anticancer Ther 2011;11:893-9. [Crossref] [PubMed]
- Minervini A, Serni S, Tuccio A, et al. Local recurrence after tumour enucleation for renal cell carcinoma with no ablation of the tumour bed: results of a prospective single-centre study. BJU Int 2011;107:1394-9. [Crossref] [PubMed]
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2007;51:1606-15. [Crossref] [PubMed]
- Adamakis I, Koutalellis G, Mitropoulos D, et al. Enucleoresection for the elective treatment of small renal cell carcinoma: can it be the treatment of choice? Onkologie 2007;30:97-102. [PubMed]
- Di Maida F, Campi R, Lane BR, et al. Predictors of Positive Surgical Margins after Robot-Assisted Partial Nephrectomy for Localized Renal Tumors: Insights from a Large Multicenter International Prospective Observational Project (The Surface-Intermediate-Base Margin Score Consortium). J Clin Med 2022;11:1765. [Crossref] [PubMed]
- Minervini A, Campi R, Sessa F, et al. Positive surgical margins and local recurrence after simple enucleation and standard partial nephrectomy for malignant renal tumors: systematic review of the literature and meta-analysis of prevalence. Minerva Urol Nefrol 2017;69:523-38. [PubMed]
- Antonelli A, Furlan M, Sodano M, et al. External histopathological validation of the surface-intermediate-base margin score. Urol Oncol 2017;35:215-20. [Crossref] [PubMed]
- Czerny H. UeberNierenexstirpation. 1890;6:484-6.
- McDougall EM, Elbahnasy AM, Clayman RV. Laparoscopic wedge resection and partial nephrectomy--the Washington University experience and review of the literature. JSLS 1998;2:15-23. [PubMed]
- Janetschek G, Abdelmaksoud AA. Laparoscopic wedge resection for renal cell carcinoma. Curr Opin Urol 2003;13:203-8. [Crossref] [PubMed]
- Choi JE, You JH, Kim DK, et al. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol 2015;67:891-901. [Crossref] [PubMed]
- Baio R, Molisso G, Caruana C, et al. "To Be or Not to Be Benign" at Partial Nephrectomy for Presumed RCC Renal Masses: Single-Center Experience with 195 Consecutive Patients. Diseases 2023;11:27. [Crossref] [PubMed]
Cite this article as: Gomes DC, Orellana FM, Zanotti RR, Zequi SDC. Nephron-sparing surgery for small renal masses: a narrative review. AME Med J 2023;8:33.

