
Esophageal perforation in Zollinger-Ellison syndrome and the importance of assessing for aorto-gastric fistula: a report of two rare cases
Highlight box
Key findings
• Two patients with Zollinger Ellison syndrome (ZES) presented acutely with esophageal perforation, at a single institution. Both patients underwent primary repair, though suffered post-operative complications; in one case, an aorto-gastric fistula and in the other, an esophageal stricture requiring colonic interposition for esophageal replacement.
What is known and what is new?
• Esophageal perforation is a rare complication of ZES. Few cases have been documented on this topic.
• A description of the management of esophageal perforation in two patients with ZES is provided.
What is the implication, and what should change now?
• This study highlights the importance of including esophageal perforation and aorto-gastric fistula in the differential diagnosis of patients with ZES presenting acutely.
Introduction
Gastrinomas are gastrin-secreting functional neuroendocrine neoplasms that lead to gastric acid hypersecretion by parietal cells in the stomach. The incidence of gastrinomas is reported at 0.5–2 cases/million population (1). Approximately 80% are sporadic, while the remainder arise in patients with multiple endocrine neoplasia type 1 (MEN1) (2). Affected patients may develop gastroesophageal reflux disease (GERD), recurrent peptic ulcers, and chronic diarrhea, a clinical syndrome termed Zollinger-Ellison syndrome (ZES) (1,2). ZES diagnosis is often hindered by inadequate specificity of its symptoms, widespread use of proton pump inhibitors, and limited availability of certain diagnostic tools (1,2). The frequency of esophageal complications such as peptic esophageal disease, strictures, and Barrett’s esophagus in patients with ZES have been prospectively studied in the past (3). However, reports of esophageal perforation, are uncommon.
In a retrospective review of 24 patients with ZES by Bondeson and colleagues (4), the frequency of substantial esophageal involvement was examined. Eighteen patients had an endoscopic examination of their esophagus prior to gastrinoma resection. Of those patients, 13 had evidence of esophagitis, of which 9 had ulceration. Three patients went on to develop severe esophageal complications including massive bleeding from an esophageal ulcer, severe strictures and one case of perforation following stricture dilatation. There have been few case reports of esophageal perforation occurring in ZES patients (5,6). These patients all had a peptic strictures and perforation occurred following endoscopic dilatation or after vomiting. Due to sparse literature on the topic, it is unclear whether there are unique perioperative considerations surgeons should be aware of for the tailored care of ZES patients with esophageal perforation.
Herein, we present two cases of acute esophageal perforation following emesis, in middle aged females with ZES. One of these patients developed an aorto-enteric fistula secondary to ZES-related peptic ulcer disease. To date, there is no current literature on this potential complication of ZES. The objective of this work is to describe the diagnosis and management of these patients, while highlighting the unique post-operative complications encountered in their treatment. We present both cases in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-23-48/rc).
Case presentation
Description of case 1
A 49-year-old female presented to her community emergency department (ED) with new onset nausea, vomiting, and abdominal pain. Figure 1 shows the timeline for her care. Her past medical history was significant for suspected ZES (she had been lost to follow-up), severe esophagitis, gastritis, and duodenitis with recurrent gastrointestinal (GI) bleeds, and chronic abdominal pain. Her family’s medical history was negative for endocrinopathies, though her mother had lung cancer and father had colorectal cancer. Chest X-ray (CXR) at presentation to the ED revealed a large right-sided tension hydropneumothorax. Subsequent computed tomography (CT) scan showed perforation of the distal esophagus at the gastroesophageal junction (GEJ) with an open communication of the GI tract to the right pleural space. She was transferred urgently to a tertiary care center. On arrival, she was stable and in no destress. She was borderline tachycardic, normotensive and had an oxygen saturation of 97% on 12 liters/minute (L/min) of oxygen.
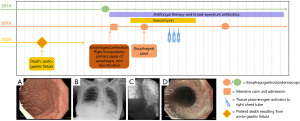
She was taken to the operating room (OR) urgently where a right thoracotomy, decortication, repair of esophagus, and esophagogastroduodenoscopy (EGD) were performed. There was a significant pleural effusion with fibrin on the lung. The lung was decorticated to allow re-expansion. In the distal esophagus, there was a perforation extending from 3 cm above the GEJ and 3 cm distally into the proximal stomach. The esophageal tissue was fibrotic, consistent with a chronic stricture. On the stomach, a plane between the muscularis and the mucosa was easily established but, despite multiple attempts, could not be developed on the esophageal side of the perforation. The gastric portion of the perforation was closed in two layers with absorbable sutures, while the tissues composing the fibrotic stricture on the esophageal side were re-approximated in one layer. An air leak test using water was negative for a persistent leak. An intercostal muscle flap was used to buttress the repair. Two chest tubes were used to drain the pleural space and a Jackson-Pratt drain was placed near the anastomosis.
Postoperatively, she was transferred to the intensive care unit (ICU) in stable condition and started on piperacillin-tazobactam 2.25 g intravenous (IV) q6h (a loading dose of 3.375 g was administered in the OR) and caspofungin 50 mg IV q24h, as well as total parenteral nutrition (TPN). Pleural fluid and blood cultures grew Candida albicans. The patient underwent investigations for fungal endocarditis and endophthalmitis, which were negative.
On postoperative day (POD) 6, a barium swallow study with water-soluble contrast showed a leak in the distal esophagus. The patient remained clinically stable. The leak was managed with placement of an esophageal stent (100 mm × 20 mm Taewoong stent, Cook Medical, New York, USA). A repeat swallow study on POD 8 no longer demonstrated a leak.
The patient developed sepsis from a residual empyema which was managed with tissue plasminogen activator (tPA) and a percutaneous drain. Culture of the pleural fluid grew Candida tropicalis. By POD 27, the patient was tolerating a full fluids diet.
Unfortunately, on POD 45, she had an episode of hematemesis followed by sudden cardiac arrest. She was pronounced dead after 25 minutes of cardiopulmonary resuscitation (CPR) with no return of spontaneous circulation. A large amount of blood was found in her trachea and in the chest tube, after the attempted resuscitation.
An autopsy was performed. A 2.0-cm gastrinoma was identified in the duodenal bulb. Regional lymph node metastases were present, and there were innumerable liver metastases, all less than 1.5 cm. The stomach was enlarged and had a thickened wall with prominent rugae and parietal cell hypertrophy and hyperplasia, consistent with ZES. There were numerous erosions and ulcers in the duodenal and gastric mucosa. Associated with a gastric ulcer, there was an aorto-gastric fistula, involved by invasive candidiasis. Tissue bank culture collected at post-mortem procurement grew Candida tropicalis.
Description of case 2
A 48-year-old female presented to her community ED with sudden onset pleuritic chest pain, following an episode of vomiting. Figure 2 shows the timeline for her care. The patient’s history was relevant for MEN1, for which she had previously undergone four-gland parathyroidectomy, laparoscopic distal pancreatectomy, and splenectomy for a gastrinoma [4 tumors, pT1(m)N0], right lower lobectomy for carcinoid tumor, and multiple EGDs with dilatations for chronic esophageal stricture. She had no family history of endocrinopathy, thyroid cancer, carcinoid tumor, hyperparathyroidism, or any disease to suggest MEN1. On assessment, a CXR demonstrated a right-sided hydropneumothorax. A chest tube was inserted and drained 2.5 L of dark maroon fluid. The patient was transferred to a tertiary center. On arrival, she was hypotensive, tachycardic, and requiring 2 L/min of oxygen. Blood work revealed the presence of acute kidney injury. A CT scan showed distal esophageal perforation with open communication to the right pleural space. Due to bloody output from the chest tube, a computed tomography angiogram (CTA) was performed to assess for aorto-gastric fistula. This was negative.
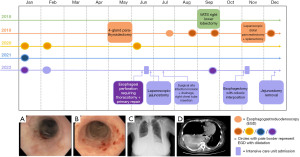
The patient was urgently taken to the OR. A right posterolateral thoracotomy was performed, and purulent fluid was removed from the pleural space. A distal esophageal perforation was identified. This appeared to have occurred through fibrotic tissue where there was no distinct muscularis. The defect was closed primarily. A serratus flap was performed to reinforce the repair. A Jackson-Pratt drain was placed in the bed of the serratus muscle. A nasogastric (NG) tube was inserted, along with two right-sided chest tubes. An EGD was performed at the end of the case. The scope could not pass beyond the repair. Postoperatively, she was started on piperacillin-tazobactam 3.375 g IV q6h, fluconazole 400 mg IV daily, and TPN. Pleural fluid grew methicillin-sensitive Staphylococcus aureus (MSSA) and mixed anaerobes. She was subsequently started on vancomycin 750 mg IV two times a day (BID).
On POD 5, the patient returned to the OR and underwent laparoscopic jejunostomy tube (J-tube) placement. Post-operatively, she did well, though required physiotherapy for deconditioning and ongoing IV antibiotics (ceftriaxone 1 g IV daily and flagyl 500 mg IV q12h). On POD 20, she was discharged home on J-tube feeds and IV antibiotics. She later returned to hospital with nausea/vomiting and associated leukocytosis. She was found to have a right-sided empyema and a deep wound infection at the site of the right serratus muscle flap harvest. She underwent tPA to the right pleural space, and operative drainage of the periscapular wound with vacuum-assisited closure (VAC) dressing application.
Following this treatment, she did well, through remained J-tube feed dependent secondary to severe persistent stricturing at the site of the perforation. The option of esophagectomy with colonic interposition was discussed and the patient opted to pursue this surgery. A right posterior-lateral thoracotomy allowed intra-thoracic mobilization of the esophagus and proximal stomach and release of the serratus anterior flap that was used to buttress the perforation. In the supine position, a laparotomy and left neck incision facilitated mobilization and resection of the entire stomach and majority of the cervical esophagus. The colon was mobilized preserving the left colonic vascular pedicle to allow isoperistaltic interposition of the colon to replace the esophagus. Aside from one episode of mucus plugging requiring 12 hours of intubation and bronchoscopy for respiratory distress, the patient recovered well and was discharged home. Interestingly, she developed a subcutaneous abscess at her J-tube site, following its removal. This was treated with debridement and a VAC dressing. She continues to undergo surveillance with annual chest CT for her history of carcinoid tumor. Most recent imaging has been negative for regional recurrence or any other systemic disease. Her main issue has been around nutrition. She is underweight (body mass index of 18.1 kg/m2) and is being followed by a dietician for an aversion to food. Weight gain will be the focus of her ongoing care.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). For both cases, written informed consent was obtained from the patient’s substitute decision maker or the individual themselves, for publication of this case report and accompanying images which are also declassified from patient identifiers. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In this report, two patients with ZES presented acutely with esophageal perforation. Both patients had known esophageal strictures, which limited the primary repair of the esophageal defect, as it could not be performed in two layers. Post-operatively, one patient suffered from candidemia, an anastomotic leak managed with stenting, and a delayed massive hemorrhage from an aorto-gastric fistula. The second patient required a J-tube for nutritional support and later required colonic interposition for esophageal replacement. Both patients were treated for an empyema in the post-operative period.
This report has several strengths. Though esophageal perforation in ZES is considered a rare pathology, this report shows that it has been observed at a single institution on two occasions only a couple years apart. The entire peri-operative course of both patients has been described, whereas previous case reports lack certain valuable information around diagnosis and treatment of patients with ZES who experience esophageal perforation. This work also presents an outcome, an aorto-enteric fistula, which may be unique to patients with ZES. This work is nevertheless limited by its observational nature as a case report, restricting generalizability to all patients with ZES.
Current guidelines recommend patients with esophageal perforation should undergo a two-layer repair with separate suturing of the mucosa and muscle (7). When performing a primary repair, buttressing the repair with surrounding viable tissue has been shown to decrease the risk of leakage (7,8). Patients who suffer large esophageal perforations with extensive extraluminal contamination, in the setting of underlying malignancy, non-dilatable esophageal structure, or achalasia, are recommended to undergo esophageal diversion with or without esophagectomy (8).
In accordance with these recommendations, Ng and colleagues described a patient who had spontaneous esophageal perforation during admission for dysphagia, secondary to an esophageal stricture that could not be traversed endoscopically (5). They subsequently underwent a subtotal esophagectomy with gastric conduit reconstruction. Seven days after surgery, the patient developed a leak, from the gastric conduit, while esophagogastric anastomosis remained intact. A subtotal gastrectomy with esophagostomy was performed. Surgical pathology disclosed two perforated gastric ulcers and further imaging identified a gastrinoma in the uncinate process of the pancreas, which was later successfully resected, at the same time as completion gastrectomy, and esophageal reconstruction with colon interposition.
Ruttan and Sanders describe an atypical presentation of esophageal rupture following an emetic event, in a patient suspected of having ZES who had a known esophageal stricture previously treated with balloon dilatations (6). This patient was found to have bilateral perforation of the esophagus and underwent thoracotomy and exploratory laparotomy. The authors do not describe findings of that initial operation, though one week later, the esophagus was repaired, and a feeding J-tube was inserted. In this case, definitive diagnosis of ZES is not described, nor an explanation for delayed surgical repair or the reason for the patient’s prolonged post-operative course.
Esophageal perforation in the setting of ZES is rare and offers unique challenges to the management of the patient. Thus, the reported cases emphasize that clinicians should consider the differential diagnosis of ZES in the setting of benign peptic esophageal strictures with perforation. In the first case, a gastrinoma had not been located or resected at the time of the perforation despite several pre-mortem imaging studies and upper endoscopy, therefore increasing her risk of high gastric acid in the postoperative period and recurrent ulcers. Although our patient’s repair initially leaked, this was salvaged with an esophageal stent. More aggressive maneuvers such as esophagectomy in this setting are not appealing, particularly with a suspect gastrinoma, as was highlighted in the case by Ng et al. (5). Consideration of stent placement at the time of initial repair may have prevented the leak and the subsequent empyema, however, the second case highlights that esophageal stenting was not required to prevent a leak, though similar conditions were present. Utilization of an esophageal stent at the time of the initial operation is a challenging surgical decision. Several factors should be considered by the operating surgeon: (I) the condition of the esophagus and stomach; (II) the degree of contamination in the local environment; (III) the patient’s acute metabolic status; and (IV) the patient’s past medical history. The surgeon must consider the risks of stent placement, including ischemia at the site of the repair secondary to the radial pressure from the stent and additional operating time to place the stent in an acutely ill patient, against the probability of the repair healing and the patient’s ability to tolerate a leak.
Unfortunately, our first patient died awaiting transfer to her home hospital of a massive bleed from an aorto-gastric fistula secondary to her underlying disease, and complicated by invasive Candida. An aorto-gastric fistula at the surgical site is a known complication of surgery around the GEJ or invasive carcinoma (9-11), but in this case, the surgical site remained intact. This exceedingly rare complication from ZES is important to recognize and could alter survival in these patients. As in the second case, when blood is noted from a chest tube following esophageal perforation, a CTA can be used to assess for aorto-gastric fistula, as this would alter management.
Conclusions
In summary, the two cases herein demonstrate that esophageal perforation should be recognized as a possible complication of ZES, in patients with esophageal stricture. Timely recognition of this condition is required, as significant morbidity, and even mortality, may be associated with esophageal rupture. Though rare, clinicians should recognize the risk of aorto-gastric fistula in this patient population.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-23-48/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-48/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). For both cases, written informed consent was obtained from the patient’s substitute decision maker or the individual themselves, for publication of this case report and accompanying images which are also declassified from patient identifiers. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chatzipanagiotou O, Schizas D, Vailas M, et al. All you need to know about gastrinoma today | Gastrinoma and Zollinger-Ellison syndrome: A thorough update. J Neuroendocrinol 2023;35:e13267. [Crossref] [PubMed]
- Rossi RE, Elvevi A, Citterio D, et al. Gastrinoma and Zollinger Ellison syndrome: A roadmap for the management between new and old therapies. World J Gastroenterol 2021;27:5890-907. [Crossref] [PubMed]
- Hoffmann KM, Gibril F, Entsuah LK, et al. Patients with multiple endocrine neoplasia type 1 with gastrinomas have an increased risk of severe esophageal disease including stricture and the premalignant condition, Barrett's esophagus. J Clin Endocrinol Metab 2006;91:204-12. [Crossref] [PubMed]
- Bondeson AG, Bondeson L, Thompson NW. Stricture and perforation of the esophagus: overlooked threats in the Zollinger-Ellison syndrome. World J Surg 1990;14:361-3; discussion 363-4. [Crossref] [PubMed]
- Ng T, Maziak DE, Shamji FM. Esophageal perforation: a rare complication of Zollinger-Ellison syndrome. Ann Thorac Surg 2001;72:592-3. [Crossref] [PubMed]
- Ruttan TK, Sanders AB. "Paramedics are bringing in a hypotensive gastrointestinal bleeder": an unexpected diagnosis. J Emerg Med 2012;43:e227-9. [Crossref] [PubMed]
- Chirica M, Kelly MD, Siboni S, et al. Esophageal emergencies: WSES guidelines. World J Emerg Surg 2019;14:26. [Crossref] [PubMed]
- Khaitan PG, Famiglietti A, Watson TJ. The Etiology, Diagnosis, and Management of Esophageal Perforation. J Gastrointest Surg 2022;26:2606-15. [Crossref] [PubMed]
- Kougias P, Baltazar U, Battle WJ, et al. Primary aortogastric fistula after nissen fundoplication: a case report and review of pertinent literature. Vasc Endovascular Surg 2003;37:135-9. [Crossref] [PubMed]
- Li M, Li Y, Zhu Q, et al. Primary Aortogastric Fistula Caused by Ulcerated Gastric Carcinoma: A Rare Cause of Sudden Death. J Emerg Med 2020;58:e169-71. [Crossref] [PubMed]
- Maria T, Maria A, Dimitrios V, et al. A rare case of sudden death due to aortogastric fistula after endovascular aortic repair of the descending aorta. Forensic Sci Med Pathol 2021;17:461-4. [Crossref] [PubMed]
Cite this article as: Robinson AM, McNeil MV, Stueck AE, Ermerak NO, Plourde M, French DG. Esophageal perforation in Zollinger-Ellison syndrome and the importance of assessing for aorto-gastric fistula: a report of two rare cases. AME Med J 2023;8:39.

