
Integration of robotic bronchoscopy and cone beam computed tomography: a narrative review
Introduction
The last decade in medicine has seen a revolution in technology available for the diagnostic evaluation of pulmonary nodules. Historically, nodules less than 2 cm or in the outer third of the lung parenchyma have been difficult to reach by conventional bronchoscopy even with the use of technologies such as radial-endobronchial ultrasound (r-EBUS), thin and ultrathin bronchoscopes (1-4). What used to be a field dominated by fluoroscopy-guided bronchoscopy for large central lesions and the use of transthoracic needle biopsy (TTNB) for peripheral nodules, has seen the advent of both hardware improvements and advanced imaging integration. Robotic technology combined with electromagnetic or shape-sensing navigation aims to provide stability, precise control, and more distal airway cannulation. Cone beam computed tomography (CBCT) during bronchoscopy allows for tool-in-lesion confirmation using three-dimensional (3D) reconstruction and/or CBCT derived augmented fluoroscopy (AF). There are other computer-based systems that allow for standalone AF technology utilizing traditional C-arms. These advanced imaging techniques provide superimposed guidance on live fluoroscopy to steer biopsy tools towards the target.
Both CBCT and robot-assisted navigational bronchoscopy (RANB) allow for navigation to and biopsy of smaller, more peripheral nodules bronchoscopically (5). While TTNB has reported diagnostic yield (DY) reaching 91%, it has also been associated with a 10% risk of pneumothorax (6). More recent literature suggests that patients undergoing TTNB for cancer diagnosis are at increased risk for ipsilateral pleural recurrence due to tumor seeding of the biopsy track (7). Furthermore, TTNB in the traditional radiology setting does not allow for invasive mediastinal staging at the time of initial pulmonary nodule sampling as is often indicated (8). This has set the stage for innovation as we look toward minimally invasive technologies that have fewer adverse events, improve DY closer to that of TTNB, and allow for simultaneous lymph node staging.
Increasing effectiveness of minimally invasive nodule diagnosis has become all the more important as more patients are identified with pulmonary nodules through guideline recommended lung cancer screening (9). The goal of newer technology is 2-fold. First, increased precision theoretically offers improved DY; correctly identifying the true positive and true negative cases (10). Second—as diagnostic methods continue to evolve, increased precision in navigation will provide for a possible therapeutic application of navigational bronchoscopy such that lung cancer treatment itself could include the use of these technologies to provide tumor directed therapeutics that reduce lung cancer morbidity and mortality (11). However, DY varies amongst these technologies and minimal large prospective data exists to support the effectiveness of these newer methods. The objective of this review is to discuss the current body of evidence for these new technologies in diagnostic bronchoscopy, and highlight how a combination of these technologies could provide an increased DY of navigational bronchoscopy. We present this article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-23-96/rc).
Methods
The authors conducted a literature review using online database resources, primarily PubMed, searching from 2010 to 2023. We reviewed all peer reviewed studies published with the search criteria listed in Table 1. Descriptive epidemiology studies (i.e., case series), randomized clinical trials, structured reviews, and meta-analyses were considered for inclusion. All studies included in this review article had to provide their study DY and their DY definition.
Table 1
| Items | Specification |
|---|---|
| Date of search | 04/01/2023 |
| Databases and other sources searched | PubMed, Ovid Medline, GoogleScholar |
| Search terms used | Electromagnetic navigational bronchoscopy, cone beam CT and bronchoscopy, robot assisted bronchoscopy, augmented fluoroscopy and bronchoscopy, shape sensing robotic navigation, trans-thoracic needle aspiration, radial endobronchial ultrasound |
| Timeframe | 2010-2023 |
| Inclusion and exclusion criteria | All case series, retrospective, prospective, and metanalysis were considered; exclusion was follow-up of long-term data of previously conducted study; studies were excluded if they did not report a diagnostic yield or their methods for calculating a diagnostic yield |
| Selection process | Review was conducted by three authors independently with consensus reached for inclusion if two authors felt a study was appropriate |
CT, computed tomography.
Diagnostic accuracy (DA) versus DY
DY, the commonly used term in interventional bronchoscopy literature, refers to the rate of a positive or clinically definitive finding for a given test. Whereas, a less commonly used term, the DA, uses the sensitivity and specificity of a test within a study population, and evaluates the proportion of correctly classified results for a given disease (i.e., lung cancer). Therefore, DA has a standardized definition and permits the calculation of sensitivity, specificity, positive predictive value, and negative predictive value. DY, on the other hand, has a wide range of definitions and introduces the idea of integrating follow up testing into the definition of a true negative result (12). The existing literature on bronchoscopy for the diagnosis of a pulmonary nodule has a wide range of DA and DY reported with many versions of DY used. This leads to significant variation in the DY of bronchoscopy according to the definition used, and the inclusion of follow up information into the DY calculation (10). The DY can be altered based on how often benign, non-specific, or non-diagnostic biopsy results are categorized as true negatives, and how patients lost to follow up are handled. For this review, we have categorized the included studies by DY methodology (Table 2) as described below.
Table 2
| Device | Outer sheath diameter | Data supporting use | Disposables | Navigation system | Technology highlights | Potential cons to technology |
|---|---|---|---|---|---|---|
| Monarch | Outer sheath: 6 mm; inner scope: 4.2 mm |
Multiple single-arm studies in humans, including multicenter studies | Compatible with tools via 2.0 mm working channel | EMN augmented by robotic data and computer vision | Vision is maintained, mother-daughter scope design | Large outer diameter, known limitations to EMN |
| Galaxy | 4.0 mm | Porcine study only | Compatible with tools via 2.0 mm working channel | EMN plus proprietary digital tomosynthesis (TiLT Technology) | Integrated augmented fluoro and RAB system with real time update in nodule location, vision is maintained, disposable single use bronchoscope | Limits to tomogram-derived imaging and EMN, limited in-human peer-reviewed data |
| Ion | 3.5 mm | Multiple single-arm studies in humans, including multicenter studies | Built to use with proprietary TBNA needles made by Intuitive, compatible with tools via 2.0 mm working channel | Shape sensing technology | Small outer diameter 3.5 mm, shape sensing not affected by C-arm interference | Vision is lost when using biopsy tools, unique scope reprocessing takes place on site which can create a burden for staff |
EMN, electromagnetic navigation; RAB, robotic assisted bronchoscopy; TBNA, transbronchial needle aspiration.
A strict categorization of DY only includes data available at the time of bronchoscopy, meaning only specific diagnoses made via bronchoscopy can be categorized as diagnostically successful. Using this strict definition of DY, there is no role for adjudicating non-specific or non-diagnostic biopsies as true negatives using follow up data. An intermediate category allows for the inclusion of follow up data only for non-specific benign biopsy results, and allows for categorization of nonmalignant diagnoses as true negatives if confirmed by a subsequent biopsy or the evolution of follow up imaging. A liberal category allows for follow up of all biopsies initially negative for malignancy, including non-diagnostic samples showing normal pulmonary elements, and categorizes them as true negatives if a subsequent biopsy confirms nonmalignant diagnosis or imaging evidence of benign disease. In the intermediate and liberal categories, patients with non-malignant biopsy results who are lost to follow up can be either excluded from the analysis, counted as true negatives, or counted as false negatives.
Discussion
Bronchoscopy literature variation
Evaluation of new technology has been plagued by many factors. As most data comes from single arm prospective or retrospective cohorts, these studies are prone to bias. Two of the most prominent biases are selection bias and measurement bias. Selection bias in these studies is driven by patient selection for ideal candidates for emerging diagnostic tools (13). Frequently, patients are not enrolled consecutively and those not deemed good candidates for new technology are excluded from studies (14). As mentioned previously, the definition of DY used by studies varies widely as well, which can exaggerate or underestimate study results, causing measurement bias. An example can be found in the two largest data sets in the diagnosis of peripheral pulmonary nodules (PPNs) that have evaluated electromagnetic navigation (EMN) technology. In 2016, the AQuIRE registry published observational comparisons of EMN to bronchoscopy with and without r-EBUS. They found a DY of 63.7% when bronchoscopy alone was used compared to a DY of 57% for bronchoscopy with r-EBUS and a DY of 38.5% when EMN was used (15). More recently the NAVIGATE study demonstrated a DY of 73% for EMN in a prospective cohort of 1,215 patients (5). The AQuIRE registry used a strict DY definition where only categorization of pathology at the time of the biopsy was included in determining DY, whereas in NAVIGATE a more liberal definition of DY was used that allowed for the recategorization of non-diagnostic samples. The previously mentioned biases are two main issues with both multi and single arm studies in diagnostic bronchoscopy. Multi-arm observational studies also have issues with another bias, confounding by indication. Confounding by indication is when the cases with the most difficult navigation are selected into the arm with the technology that has the perception of the highest probability of success and therefore the DY of that arm could be artificially low compared to older technology. More recent literature has attempted to evaluate the overall effectiveness of diagnostic bronchoscopy and comparative effectiveness of diagnostic bronchoscopy technology, attempting to overcome the variation historically seen in the literature.
Two systematic review and meta-analyses have been published on this topic. Nadig et al. found an overall DY for guided bronchoscopy of pulmonary lesions to be 69.4%. They found no difference in DY in studies prior to 2012 compared to after 2012, suggesting no improvement in DY with advances in technology (16). In subgroup analysis, there was no difference in DY when newer technology was added, though the limitation of this study is that all new technologies such as CBCT, RANB, and AF were grouped into a single category in subgroup analysis. Kops et al. published a similar meta-analysis using different study selection criteria, and found an overall DY of 70.9%. This study did do subgroup analysis of technologies used and found that newer techniques, including CBCT, RANB, and tomosynthesis based EMN have a statistically higher DY than older techniques, suggesting that there has been some improvement in DY when evaluating the newest technology (17). Kops et al. also categorized studies by the definition used by Vachani et al. (10) but found no statistical difference in DY when comparing studies that used a liberal definition of DY to those that used a strict definition. However, both of these studies comment on the significant study heterogeneity, which is primarily due to the variation in study design and DY definition.
Another area of variation in bronchoscopic literature has been the type of biopsy tool used in diagnosing PPNs. The AQuIRE registry showed the marginal benefit of peripheral transbronchial needle aspiration (TBNA) when added to transbronchial biopsy with forceps, bronchoalveolar lavage, and brushings to be 6.3%, but that must be weighed against the marginal risk incurred by performing needle aspiration (18). The added benefit of TBNA remains to be studied in a prospective randomized fashion. The author in this response notes a significant amount of resources would have to be used to answer this question appropriately. More recently, cryobiopsy is being considered for the diagnosis of PPNs. It has not been prospectively evaluated, however, ongoing clinical trials should help clarify the utility of this emerging technology (NCT05751278).
Traditional navigational bronchoscopy
EMN bronchoscopy and virtual bronchoscopic navigation (VBN) have been used in combination with r-EBUS to reach more PPNs. These modalities use the same basic concept: generate a 3D roadmap of the bronchial tree and provide directionality to the bronchoscopist to enhance biopsy accuracy and precision. These modalities use software to analyze computed tomography images obtained prior to bronchoscopy. This guidance ranges from VBN where a simulated airway path to the nodule of interest is created; to EMN where an electromagnetic signal from instruments placed inside the lung provide 3D spatial information about the instrument’s relationship to the lesion. Initial results of navigational software for the diagnosis of pulmonary nodules demonstrated positive results. A meta-analysis by Jiang et al. (3) supported the use of navigational bronchoscopy as it increased DY by an odds ratio (OR) of 1.69 [confidence interval (CI): 1.32–2.18, P<0.001] for pulmonary nodules. The pooled DY of navigational bronchoscopy was 73% compared to 62% for non-navigational bronchoscopy. This meta-analysis supported the use of navigation as a tool to increase DY in more peripheral, smaller lesions, with a bronchus sign. Another meta-analysis published by Giri et al. found no difference in DY between VBN compared to non-VBN, 74.17% to 69.51%, however, VBN had superior DY for pulmonary nodules <2 cm with a risk ratio (RR) of 1.18 (95% CI: 1.05–1.32) (19). We must interpret these analyses with caution, as the biases and methodologic issues mentioned above do apply. For example, more recently a prospective study using a strict definition of DY found that EMN alone only had a DY of 53% (20). This study demonstrates possible limitations of EMN using a strict DY definition with prospective, careful data collection in a multi-centered setting.
As the initial navigational technology has emerged, it is worth mentioning that VBN offers a more cost-effective option. VBN does not consume disposables or require the purchase of extensive hardware beyond the traditional guidance of r-EBUS. VBN has performed well when combined with r-EBUS even in the prospective setting (1,21-23). However, this technology, as well as other virtual and EMN based techniques are limited by CT -to-body divergence, differences between the pre-procedure CT mapping and the true anatomy of the PPN during bronchoscopy (24). CT-to-body divergence is affected by factors including atelectasis, lung volume during mechanical ventilation versus awake breath holding during pre-procedure imaging studies, nodule movement during respiration, and anatomic changes in the time from the planning CT to the procedure (25,26). Specifically, in EMN, a study found that on average a pulmonary nodule moved 17.6 mm between end exhalation and full inspiration, and that nodules located in the lower lobes moved more than in upper lobes (26). In an effort to reduce or eliminate CT-to-body divergence, newer technologies discussed below are less dependent on pre-procedural CT scan analysis in an effort to improve accuracy (24).
CBCT and AF
CBCT involves cross sectional imaging intra-procedurally using a capable C arm in the bronchoscopy suite. This is traditionally done in a hybrid OR using fixed CBCT systems. The use of CBCT to increase DA/DY in pulmonary nodule diagnosis is twofold. First, CBCT could demonstrate a biopsy tool within the lung nodule (“tool-in-lesion”)—allowing the bronchoscopist to radiographically demonstrate successful navigation and placement of a biopsy instrument into a pulmonary nodule. This may or may not significantly increase DA/DY despite the obvious plausible benefit. Casal et al. studied the concept of CBCT tool-in-lesion in a prospective analysis of 20 patients and demonstrated a DY of 70%, similar to that provided by EMN or VBN alone (27). However, Casal et al. and other CBCT literature has shown a navigational yield, where the biopsy instrument is demonstrated to be within the nodule, to be up to 91%, suggesting the difference between navigational yield and biopsy yield may be limitations intrinsic to the biopsy instruments available (28). This aspect of navigational yield becomes valuable when considering the therapeutic application of bronchoscopy in the future for the treatment of lung cancer, where accurate placement of an ablative tool within the lesion is critical.
The second important effect of CBCT on DA/DY is the combination of CBCT with AF to improve navigation to pulmonary nodules using an image that is much less susceptible to CT-to-body divergence. AF allows for the overlay of a highlighted, artificial pulmonary nodule target directly onto the real-time fluoroscopy screen during biopsy. This is achieved by utilizing the CBCT imaging obtained intraoperatively along with software that translates the 3D position of the pulmonary nodule onto the 2D image of traditional fluoroscopy. This process is frequently called “segmentation” and allows a computer system to understand the position of a pulmonary nodule by referencing bone landmarks and set references within a hybrid OR (29). This highlighted target image then provides another data point for navigation and localization, decreasing the effects of atelectasis and periprocedural variation in anatomy by relying on a cross sectional image collected just moments before the biopsy starts. This information is not truly “real-time” in that the overlay is based on the CT scan that was just collected, rather than coaxial imaging happening during the actual biopsy maneuver.
Using either of these benefits of CBCT, or their combination, several single-arm descriptive studies and observational studies have shown impressive results with DY ranging from 70–90% (see Table 3) (26,35,37,38,51). A retrospective cohort study comparing CBCT + AF to r-EBUS alone found that CBCT improved DY to 75.5% from 52.8% (32). In another retrospective cohort study of 236 patients the DY of CBCT + AF was not statistically significantly different compared to r-EBUS alone (83.5% in the CBCT group versus 74.4% in the r-EBUS group, P=0.06). However, when considering smaller lesions <20 mm, CBCT with AF had a statistically significant higher DY. The authors attributed this to the improved navigation of CBCT with AF for smaller pulmonary nodules (52). A retrospective comparison study of EMN and CBCT versus EMN found increased DY of 74.2% with EMN + CBCT compared to 51.6% with EMN alone using a strict definition of DY (34). Most recently, using a strict DY definition, DiBardino et al. demonstrated a DA of 86.7% with the combination of r-EBUS, ultrathin bronchoscopy, CBCT with AF, and frequent tool-in-lesion confirmation (36). While much of this data has shown that CBCT with AF has improved DY compared to r-EBUS alone or EMN, many of these studies are prone to selection bias, confounding by indication, and lack of generalizability given their single-center, retrospective design.
Table 3
| Author | Year | Population | Modalities | Overall diagnostic yield | Study definition |
|---|---|---|---|---|---|
| EMN/VBN | |||||
| Wang Memoli et al. (30) | 2012 | Meta-analysis | “Guided bronchoscopy” included EMN, VNB, r-EBUS, ultrathin bronchoscope | 70% | Multiple |
| Folch et al. (NAVIGATE) (31) | 2019 | Prospective cohort study of 1,215 patients at 29 academic community centers | EMN | 73% | Liberal |
| Yu et al. (32) | 2021 | Retrospective consecutively matched non-randomized 308 patients | r-EBUS + CBCT with AF vs. r-EBUS alone | 75.5% vs. 52.8% | Strict |
| Thiboutot et al. (20) | 2023 | Prospective cohort study of 160 participants at 8 centers | EMNB vs. EMN-TTNA vs. EMNB + EMN-TTNA | 49% vs. 27% vs. 53% | Strict |
| CBCT + AF | |||||
| Hohenforst-Schmidt et al. (28) | 2014 | Prospective single arm analysis of 33 patients with incidental solitary pulmonary nodules | EMN, CBCT, AF | 70% | Strict |
| Pritchett et al. (33) | 2018 | Retrospective analysis of 75 patients | EMN, CBCT, AF | 83.7% | Strict |
| Kheir et al. (34) | 2021 | Retrospective two-armed comparison of 62 patients | EMN vs. EMN + CBCT | 74.2% vs 51.6% | Strict |
| Verhoeven et al. (35) | 2021 | Prospective two-armed comparison of 87 patients | CBCT + AF + r-EBUS vs. EMN + r-EBUS + tool in lesion | CBCT AF alone 61.75%, EMN 50%, combined ~70% | Liberal |
| DiBardino et al. (36) | 2023 | Comparative retrospective cohort study of 116 patients | CBCT + UTB, r-EBUS, AF vs. CBCT, r-EBUS, AF vs. r-EBUS | 86.7% vs. 70.4% vs. 42.4% | Strict |
| CBCT alone | |||||
| Casal et al. (27) | 2018 | Prospective single arm analysis of 20 patients | r-EBUS, CBCT tool in lesion | 70% | Moderate |
| Ali et al. (37) | 2019 | Prospective single arm analysis of 30 patients | VBN, CBCT with tool in lesion | 90.0% | liberal |
| Kawakita et al. (38) | 2023 | Prospective single arm study of 20 patients | CBCT + UTB + r-EBUS | 85% | Strict |
| AF alone | |||||
| Aboudara et al. (39) | 2020 | Retrospective analysis of a single center experience with 101 patients | EMN + AF vs. EMN | 79% vs. 54% | Strict |
| Pritchett et al. (40) | 2021 | Prospective single arm 51 patients | AF, lung vision | 78.40% | Strict |
| Cicenia et al. (41) | 2021 | Prospective multicenter single arm 55 patients | Lung vision | 75.40% | Strict |
| Avasarala et al. (42) | 2022 | Prospective single center study with 100 patients | Illumisite | 79%% | Intermediate |
| RANB | |||||
| Chaddha et al. (43) | 2019 | Retrospective review of 165 patients | RANB, EMN, r-EBUS | 69.1% | Strict |
| Fielding et al. (44) | 2019 | Prospective single-arm analysis of 30 patients | RANB, r-EBUS | 79.3% | |
| Chen et al. (BENEFIT) (45) | 2021 | Prospective single-arm analysis of 55 patients | RANB, r-EBUS | 74.1% | Moderate |
| Kalchiem-Dekel et al. (46) | 2022 | Prospective single-arm analysis 131 patients | ssRAB | 81.7% | Strict |
| Khan et al. (47) | 2023 | Retrospective analysis of 264 patients | RANB | 85.2% at index and 79.4% at 12 months follow up | Strict and intermediate |
| Low et al. (48) | 2023 | Retrospective analysis of 133 consecutively enrolled patients | ssRAB vs. EMN | 77% vs. 80% | Strict |
| CBCT and RANB | |||||
| Benn et al. (49) | 2021 | Prospective single-arm analysis of 52 patients | RANB, CBCT | 86% | Liberal |
| Styrvoky et al. (50) | 2022 | Retrospective analysis of 209 pulmonary nodules | ssRAB, CBCT | 91% | Intermediate |
| r-EBUS and TTNA | |||||
| DiBardino et al. (6) | 2015 | Meta-analysis | CT-TTNA | 92.1% | |
| AQuIRe Registry (15) | 2016 | – | r-EBUS | 57% | Strict |
EMN, electromagnetic navigation; VBN, virtual bronchoscopy navigation; r-EBUS, radial endobronchial ultrasound; CBCT, cone beam computed tomography; AF, augmented fluoroscopy; TTNA, transthoracic needle aspiration; UTB, ultrathin bronchoscope; RANB, robot assisted navigational bronchoscopy; ssRAB, shape sensing robot assisted bronchoscopy; CT-TTNA, computed tomography guided transthoracic needle aspiration.
One of the concerns of CBCT has been added radiation exposure to patients. Various studies have evaluated the dose of radiation exposure using CBCT and the most important factor is the number of image acquisitions. Radiation exposure varies by device and number of images per spin. A phantom study in 2014 demonstrated radiation doses of 0.98–1.15 mSv from CBCT without AF (53). More recent literature has demonstrated the learning effect associated with the use of CBCT with AF. In one study radiation doses decreased from 14.3 mSv per case to 5.8 mSv per case over a 3 year period as bronchoscopists became more adept at using the technology (54). For comparison, the radiation dose for a low dose CT in the National Lung Cancer Screening Trial (NLST) was 1.4 mSv (55). While the radiation exposure of a single CBCT spin is similar to that of a low dose CT, the risk of higher exposure with more image acquisition brings up the importance of having more data to determine if a tool-in-lesion spin increases DY enough to justify a higher radiation dose.
AF without CBCT
There is also technology that generates AF images without the use of CBCT. In digital tomosynthesis a standard C-arm produces a 3D image using multiple X-rays, that in combination with software, can produce a near co-axial image of the target lesion. An example of this technology is LungVision (Body Vision Medical Inc., Campbell, CA, USA) that utilizes software that can generate AF as well as a navigational pathway, and has shown to have a DY of 78.4% when used in a prospective single arm study. This study calculated the average CT-to-body divergence at 14.5 mm which they could correct for using AF (40). A prospective multicenter study utilizing on-site cytology analysis found the DY of this technology was 75.4%, demonstrating the effectiveness of tomosynthesis-derived AF. A second technology that utilizes AF without CBCT uses similar digital tomosynthesis is Illumisite (Medtronic, Minneapolis, MN, USA). This system uses digital tomosynthesis and combines that with EMN navigation. This system has been shown to mitigate CT-to-body divergence as well (56). A single center study of 100 patients demonstrated a DY of 83% when using Illumisite (42). As stated repeatedly, this set of data suffers from the same host of methodologic issues surrounding most navigational bronchoscopy literature.
Tomosynthesis based 3D imaging alone
Similarly, C-arm technology has evolved to harness the same principles of digital tomosynthesis to provide high quality 3D imaging for the real time evaluation of biopsy tool placement without the use of AF or CBCT (57). These advanced C-arms can obtain co-axial images intraprocedurally without needing the resources of a fixed CBCT hybrid OR. These recently launched C-arms are referred to as 3D C-arms or mobile CBCT C-arms, and generate co-axial images by rotating around the patient by +/− 100 degrees over a period of roughly 30 seconds. By combining this imaging modality with EMN, a small single arm case series demonstrated a DY of 80% (58).
Robotic bronchoscopy
The past five years in interventional pulmonology has seen the Federal Drug Administration (FDA) approval of three robotic bronchoscopy systems. The systems allow for the integration of novel bronchoscopes designed to reach more peripheral lesions, navigation systems, and a more stable platform when sampling PPN. The first system to market, the Monarch Platform (Auris Health, Inc., Redwood City, CA, USA), utilizes EMN and was retrospectively evaluated with 165 cases and found a DY of 69.1%, with a successful navigation rate of 88.6% (43). A second retrospective study used both a strict and intermediate DY definition and found an impressive DY of 85.2% vs. 79.4%, demonstrating consistency between results (47).
Prospective analysis of an alternative robotic system, Ion Endoluminal Platform (Intuitive Surgical, Sunnyvale, CA, USA), has demonstrated a high DY of 79.3%, in a single center study (44). This system uses novel shape-sensing robotic assisted bronchoscopy (ssRAB) for navigation. The ssRAB tracks the shape and motion of the airways and provides a complete view of the catheter in the airway while also providing direct visualization of the airway during navigation. Robotic and navigational control are available during biopsy under fluoroscopy. Most recently, a retrospective multicenter single arm study of ION found a DY of 81.7%, and showed that prior indicators of decreased DY (size, location, and bronchus sign) are potentially less important with this technology. They found a DY of lesions <2 cm of 69.6%, a DY of 71.1% for negative bronchus sign lesions, and a DY of 70.9% for lesions in the outer third of lung parenchyma (46). Interestingly, this system has demonstrated similarly high DY when compared to EMN with digital tomosynthesis in a strict definition retrospective study (77% vs. 80%) (48).
The newest technology, Galaxy SystemTM (Noah Medical, San Carlos, CA, USA), uses an integrated tomosynthesis technology to provide intraprocedural imaging to compensate for CT-to-body divergence and create AF. It has demonstrated successful navigation to peripheral nodules in a pig model (59).
RANB has demonstrated consistently impressive DY compared to historical controls. Theoretically, these systems improve navigation accuracy and our ability to reach smaller, more peripheral nodules. However, these forms of navigation can be limited by the same CT-to-body divergence that has been problematic for EMN and VBN modalities. As such, this initial data should be interpreted with similar caution as the bulk of the data described above. Given the further novelty of initial RANB, concerns for selection bias are even higher than average. However, there is no current prospective comparative literature between RANB and CBCT, AF, EMN or a combination of technologies.
Combined advanced imaging and robotic bronchoscopy
The DA/DY of bronchoscopy for the diagnosis of pulmonary nodules has likely improved over time, but it remains unlikely that it approaches the DY seen in TTNB. Little data exists combining the most recent technologic improvements of advanced imaging and RANB. Benn et al. prospectively evaluated the combination of ssRAB with CBCT and found a DY of 86% while using a liberal definition (49). In this study, CBCT was used to provide secondary confirmation of tool in lesion rather than using CBCT-derived AF to enhance navigation and reduce real time CT-to-body divergence intraprocedurally. In a retrospective analysis of 209 pulmonary nodules, the combination of ssRAB and CBCT with AF had a DY of 91.4% (50). To date there have been no comparative trials evaluating RANB with and without advanced imaging.
Our hybrid approach
Currently, we are utilizing a hybrid approach, combining the technology of CBCT and RANB to optimize DA/DY and operating room efficiency. The authors have access to both the Monarch and Ion RANB systems that we have combined each with the Phillips Azurion ceiling mounted fixed CBCT system (Philips, Amersterdam, NL, USA). The workflow can be seen in Figure 1. The patient is brought into a hybrid OR with a ceiling mounted CBCT that allows for the use of RANB with CBCT-derived AF. The patient is intubated with an 8.5–9.0 endotracheal tube and the EMN leads are placed on the patient. The endotracheal tube is shortened using scissors to cut the proximal end of the tube roughly in line with the patient’s nose. This is to further assist the C-arm clearance with the endotracheal tube during image acquisition, as well as optimize the robotic platform’s interface with the patient. The patient is then tightly wrapped with bedding to again facilitate CBCT C-arm clearance during image acquisition. To do this, two operating room staff place the patient’s arms against their sides and secure bedding under one arm. Then the staff cross the bedding over the anterior surface of the patient and place the patient in a semi-lateral decubitus position to tightly wrap the bedding under the patient’s opposite side. The patient is then returned to the supine position. The patient is ventilated at 8–10 cc/kg ideal body weight, with positive end expiratory pressure (PEEP) of 8–15 cmH2O to reduce atelectasis. The volume and PEEP are increased for obese patients and lesions in the dependent lung zones (60). Ten recruitment breaths are then performed at 30–40 cmH2O pressure after the airway is secured.
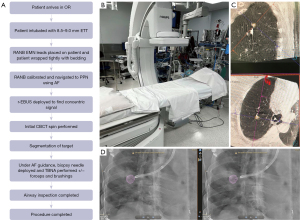
The patient is then isocentered by making sure the table height, body position on the bed, and bed position in the room permits full imaging of the region of interest on anterior-posterior and full lateral fluoroscopy imaging. CBCT C-arms utilize standard C-arm programs to check this position before acquisition of co-axial imaging. These programs are accessed via proprietary bedside controls and should be checked before connecting RANB equipment, as the bed cannot be adjusted safely after RANB devices are attached to the patient. The RANB equipment is secured to the endotracheal tube and positioned. The authors then approach the pulmonary nodule with RANB using both robotic navigation and fluoroscopy guidance. Once the nodule is potentially reached, the r-EBUS probe is inserted to further visualize the lesion. When the lesion is either located on r-EBUS, or the navigation pathway has been completed, an initial CBCT is performed. A breath hold maneuver is done during the CBCT. The pressure used for the breath hold is targeted to match the lung volume being achieved during routine ventilation at 8–10 cc/kg. This pressure will vary depending on the patient’s lung mechanics, but is generally ~5 cmH2O pressure below their peak airway pressure during ventilation. More care and precision is taken with this step for lower lobe lesions where lung movement is more dramatic (40). The initial CBCT is used to segment the lesion using software associated with the CBCT hybrid room. This allows the CBCT system to understand what target will be highlighted, and its relationship to other fixed landmarks in the patient and in the room. The segmented nodule is then overlaid onto the live fluoroscopy image (i.e., AF). Using this digital overlay, biopsy tools are then guided into the lesion. When that is achieved based on the AF image, additional CBCT spins to confirm tool-in-lesion are optional. If RANB is not available and a nodule is reached using navigational bronchoscopy, a scope holder is utilized during any further CBCT image acquisition to avoid radiation exposure to operators.
This combination of CBCT-derived AF and RANB reduces CT-to-body divergence and provides a target on live fluoroscopy to aim biopsy tools. Biopsy tool position can be checked in multiple fluoroscopy angles including angles recommended by RANB systems. As the AF guidance is not truly real-time, additional CBCT imaging to confirm tool-in-lesion has plausible benefit. If additional CBCT images do not confirm proper biopsy tool placement, the lesion can be re-segmented to create an updated AF target using the most recent CBCT, and the spatial information obtained on the CBCT can be used to reposition the biopsy tool. The use of the RANB in combination with CBCT + AF allows the authors to reach more distal pulmonary nodules and provides for a stable platform to maintain position. Many aspects of the combined procedure need further research with comparative studies. There may be little benefit in the tool-in-lesion confirmation, AF itself, and combining RANB with CBCT.
Conclusions
The past 10 years have seen a rapid increase in technology available to biopsy pulmonary nodules that has reduced barriers in nodule size and location. Current technology of CBCT and RANB have allowed for the successful navigation to PPNs over 90% of the time. With this improvement in navigation, the DY of a diagnosis for malignancy using these technologies has increased compared to historic controls, and probably ranges from 70–80% with fewer adverse events compared to TTNB. While we have seen a plateau in DY, this is in the context of the literature available. As technology has improved, bronchoscopists may be attempting to biopsy smaller and more peripheral nodules. However, without randomized or consecutive enrollment studies, trends in nodules characteristics for lesions undergoing biopsy have not been captured adequately. Little comparative data of newer bronchoscopy technology exists as most current study designs have been single arm prospective or retrospective studies often without a comparison arm. The comparative studies that do exist have been retrospective. This has provided difficulty in comparing these modalities to recommend a best practice for bronchoscopists, or support the purchase of expensive navigational or robotic systems. Future studies evaluating the cost-effectiveness of these technologies would be beneficial for understanding the impact of these technologies on the healthcare system’s costs at large. CBCT in combination with AF and RANB may provide for an appropriate workflow that is both efficient and high yield. While tool-in-lesion has demonstrated successful navigation, the subsequent improvement in DY has not been adequately studied. Any improvement in DY must be balanced by the added radiation exposure from multiple CT spins and requires further investigation. Future research should be aimed at comparative trials to best ascertain what modality or combination of modalities the field should invest in to improve DA and navigational success.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathan Kurman and Bryan S. Benn) for the series “Diagnostic & Therapeutic Bronchoscopy” published in AME Medical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-23-96/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-96/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-96/coif). The series “Diagnostic & Therapeutic Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. DD received consulting fees for product development from Olympus, Johnson & Johnson, Auris Medical and Intuitive Surgical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oki M, Saka H, Asano F, et al. Use of an Ultrathin vs Thin Bronchoscope for Peripheral Pulmonary Lesions: A Randomized Trial. Chest 2019;156:954-64. [Crossref] [PubMed]
- Kim SH, Kim J, Pak K, et al. Ultrathin Bronchoscopy for the Diagnosis of Peripheral Pulmonary Lesions: A Meta-Analysis. Respiration 2023;102:34-45. [Crossref] [PubMed]
- Jiang S, Xie F, Mao X, et al. The value of navigation bronchoscopy in the diagnosis of peripheral pulmonary lesions: A meta-analysis. Thorac Cancer 2020;11:1191-201. [Crossref] [PubMed]
- Tanner NT, Yarmus L, Chen A, et al. Standard Bronchoscopy With Fluoroscopy vs Thin Bronchoscopy and Radial Endobronchial Ultrasound for Biopsy of Pulmonary Lesions: A Multicenter, Prospective, Randomized Trial. Chest 2018;154:1035-43. [Crossref] [PubMed]
- Folch EE, Labarca G, Ospina-Delgado D, et al. Sensitivity and Safety of Electromagnetic Navigation Bronchoscopy for Lung Cancer Diagnosis: Systematic Review and Meta-analysis. Chest 2020;158:1753-69. [Crossref] [PubMed]
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis 2015;7:S304-16. [PubMed]
- Hong H, Hahn S, Matsuguma H, et al. Pleural recurrence after transthoracic needle lung biopsy in stage I lung cancer: a systematic review and individual patient-level meta-analysis. Thorax 2021;76:582-90. [Crossref] [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-20S.
- Debieuvre D, Molinier O, Falchero L, et al. Lung cancer trends and tumor characteristic changes over 20 years (2000-2020): Results of three French consecutive nationwide prospective cohorts' studies. Lancet Reg Health Eur 2022;22:100492. [Crossref] [PubMed]
- Vachani A, Maldonado F, Laxmanan B, et al. The Impact of Alternative Approaches to Diagnostic Yield Calculation in Studies of Bronchoscopy. Chest 2022;161:1426-8. [Crossref] [PubMed]
- Duke JD, Reisenauer J. Robotic bronchoscopy: potential in diagnosing and treating lung cancer. Expert Rev Respir Med 2023;17:213-21. [Crossref] [PubMed]
- Gonzalez AV, Ost DE, Shojaee S. Diagnostic Accuracy of Bronchoscopy Procedures: Definitions, Pearls, and Pitfalls. J Bronchology Interv Pulmonol 2022;29:290-9. [Crossref] [PubMed]
- Grayling MJ, Mander AP. Do single-arm trials have a role in drug development plans incorporating randomised trials? Pharm Stat 2016;15:143-51. [Crossref] [PubMed]
- DiBardino DM, Vachani A, Yarmus L. Evaluating the efficacy of bronchoscopy for the diagnosis of early stage lung cancer. J Thorac Dis 2020;12:3245-52. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68-77. [Crossref] [PubMed]
- Nadig TR, Thomas N, Nietert PJ, et al. Guided Bronchoscopy for the Evaluation of Pulmonary Lesions: An Updated Meta-analysis. Chest 2023;163:1589-98. [Crossref] [PubMed]
- Kops SEP, Heus P, Korevaar DA, et al. Diagnostic yield and safety of navigation bronchoscopy: A systematic review and meta-analysis. Lung Cancer 2023;180:107196. [Crossref] [PubMed]
- Ost DE. Reply: Quantifying the Benefits of Peripheral Transbronchial Needle Aspiration. Am J Respir Crit Care Med 2016;194:122-3. [Crossref] [PubMed]
- Giri M, Puri A, Wang T, et al. Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis. Ther Adv Respir Dis 2021;15:17534666211017048. [Crossref] [PubMed]
- Thiboutot J, Pastis NJ, Akulian J, et al. A Multicenter, Single-arm, Prospective Trial Assessing the Diagnostic Yield of Electromagnetic Bronchoscopic and Transthoracic Navigation for Peripheral Pulmonary Nodules. Am J Respir Crit Care Med 2023;208:837-45. [Crossref] [PubMed]
- Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013;188:327-33. [Crossref] [PubMed]
- Zheng X, Xie F, Li Y, et al. Ultrathin bronchoscope combined with virtual bronchoscopic navigation and endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions with or without fluoroscopy: A randomized trial. Thorac Cancer 2021;12:1864-72. [Crossref] [PubMed]
- Lachkar S, Perrot L, Gervereau D, et al. Radial-EBUS and virtual bronchoscopy planner for peripheral lung cancer diagnosis: How it became the first-line endoscopic procedure. Thorac Cancer 2022;13:2854-60. [Crossref] [PubMed]
- Pritchett MA, Bhadra K, Calcutt M, et al. Virtual or reality: divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis 2020;12:1595-611. Erratum in: J Thorac Dis 2020;12:4593-5. [Crossref] [PubMed]
- Casal RF. Cone Beam CT-Guided Bronchoscopy: Here to Stay? J Bronchology Interv Pulmonol 2018;25:255-6. [Crossref] [PubMed]
- Chen A, Pastis N, Furukawa B, et al. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest 2015;147:1275-81. [Crossref] [PubMed]
- Casal RF, Sarkiss M, Jones AK, et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis 2018;10:6950-9. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Vogl T, et al. Cone Beam Computertomography (CBCT) in Interventional Chest Medicine - High Feasibility for Endobronchial Realtime Navigation. J Cancer 2014;5:231-41. [Crossref] [PubMed]
- Setser R, Chintalapani G, Bhadra K, et al. Cone beam CT imaging for bronchoscopy: a technical review. J Thorac Dis 2020;12:7416-28. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J Thorac Oncol 2019;14:445-58. [Crossref] [PubMed]
- Yu KL, Yang SM, Ko HJ, et al. Efficacy and Safety of Cone-Beam Computed Tomography-Derived Augmented Fluoroscopy Combined with Endobronchial Ultrasound in Peripheral Pulmonary Lesions. Respiration 2021;100:538-46. [Crossref] [PubMed]
- Pritchett MA, Schampaert S, de Groot JAH, et al. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J Bronchology Interv Pulmonol 2018;25:274-82. [Crossref] [PubMed]
- Kheir F, Thakore SR, Uribe Becerra JP, et al. Cone-Beam Computed Tomography-Guided Electromagnetic Navigation for Peripheral Lung Nodules. Respiration 2021;100:44-51. [Crossref] [PubMed]
- Verhoeven RLJ, Fütterer JJ, Hoefsloot W, et al. Cone-Beam CT Image Guidance With and Without Electromagnetic Navigation Bronchoscopy for Biopsy of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol 2021;28:60-9. [Crossref] [PubMed]
- DiBardino DM, Kim RY, Cao Y, et al. Diagnostic Yield of Cone-beam-Derived Augmented Fluoroscopy and Ultrathin Bronchoscopy Versus Conventional Navigational Bronchoscopy Techniques. J Bronchology Interv Pulmonol 2023;30:335-45. [Crossref] [PubMed]
- Ali EAA, Takizawa H, Kawakita N, et al. Transbronchial Biopsy Using an Ultrathin Bronchoscope Guided by Cone-Beam Computed Tomography and Virtual Bronchoscopic Navigation in the Diagnosis of Pulmonary Nodules. Respiration 2019;98:321-8. [Crossref] [PubMed]
- Kawakita N, Toba H, Sakamoto S, et al. Cone-beam computed tomography-guided endobronchial ultrasound using an ultrathin bronchoscope for diagnosis of peripheral pulmonary lesions: a prospective pilot study. J Thorac Dis 2023;15:579-88. [Crossref] [PubMed]
- Aboudara M, Roller L, Rickman O, et al. Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: Initial experience with a novel adjunct. Respirology 2020;25:206-13. [Crossref] [PubMed]
- Pritchett MA. Prospective Analysis of a Novel Endobronchial Augmented Fluoroscopic Navigation System for Diagnosis of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol 2021;28:107-15. [Crossref] [PubMed]
- Cicenia J, Bhadra K, Sethi S, et al. Augmented Fluoroscopy: A New and Novel Navigation Platform for Peripheral Bronchoscopy. J Bronchology Interv Pulmonol 2021;28:116-23. [Crossref] [PubMed]
- Avasarala SK, Roller L, Katsis J, et al. Sight Unseen: Diagnostic Yield and Safety Outcomes of a Novel Multimodality Navigation Bronchoscopy Platform with Real-Time Target Acquisition. Respiration 2022;101:166-73. [Crossref] [PubMed]
- Chaddha U, Kovacs SP, Manley C, et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med 2019;19:243. [Crossref] [PubMed]
- Fielding DIK, Bashirzadeh F, Son JH, et al. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019;98:142-50. [Crossref] [PubMed]
- Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest 2021;159:845-52. [Crossref] [PubMed]
- Kalchiem-Dekel O, Connolly JG, Lin IH, et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022;161:572-82. [Crossref] [PubMed]
- Khan F, Seaman J, Hunter TD, et al. Diagnostic outcomes of robotic-assisted bronchoscopy for pulmonary lesions in a real-world multicenter community setting. BMC Pulm Med 2023;23:161. [Crossref] [PubMed]
- Low SW, Lentz RJ, Chen H, et al. Shape-Sensing Robotic-Assisted Bronchoscopy vs Digital Tomosynthesis-Corrected Electromagnetic Navigation Bronchoscopy: A Comparative Cohort Study of Diagnostic Performance. Chest 2023;163:977-84. [Crossref] [PubMed]
- Benn BS, Romero AO, Lum M, et al. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung 2021;199:177-86. [Crossref] [PubMed]
- Styrvoky K, Schwalk A, Pham D, et al. Shape-Sensing Robotic-Assisted Bronchoscopy with Concurrent use of Radial Endobronchial Ultrasound and Cone Beam Computed Tomography in the Evaluation of Pulmonary Lesions. Lung 2022;200:755-61. [Crossref] [PubMed]
- Chen LC, Yang SM, Malwade S, et al. Cone-Beam Computed-Tomography-Derived Augmented Fluoroscopy-Guided Biopsy for Peripheral Pulmonary Nodules in a Hybrid Operating Room: A Case Series. Diagnostics (Basel) 2023;13:1055. [Crossref] [PubMed]
- Lin CK, Fan HJ, Yao ZH, et al. Cone-Beam Computed Tomography-Derived Augmented Fluoroscopy Improves the Diagnostic Yield of Endobronchial Ultrasound-Guided Transbronchial Biopsy for Peripheral Pulmonary Lesions. Diagnostics (Basel) 2021;12:41. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Banckwitz R, Zarogoulidis P, et al. Radiation Exposure of Patients by Cone Beam CT during Endobronchial Navigation - A Phantom Study. J Cancer 2014;5:192-202. [Crossref] [PubMed]
- Verhoeven RLJ, van der Sterren W, Kong W, et al. Cone-beam CT and Augmented Fluoroscopy-guided Navigation Bronchoscopy: Radiation Exposure and Diagnostic Accuracy Learning Curves. J Bronchology Interv Pulmonol 2021;28:262-71. [Crossref] [PubMed]
- Larke FJ, Kruger RL, Cagnon CH, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol 2011;197:1165-9. [Crossref] [PubMed]
- Pritchett MA, Bhadra K, Mattingley JS. Electromagnetic Navigation Bronchoscopy With Tomosynthesis-based Visualization and Positional Correction: Three-dimensional Accuracy as Confirmed by Cone-Beam Computed Tomography. J Bronchology Interv Pulmonol 2021;28:10-20. [Crossref] [PubMed]
- van Walsum T, van de Kraats EB, Voormolen MH, et al. Navigation with three-dimensional rotational radiographic data for transpedicular percutaneous needle introduction: feasibility and comparison with fluoroscopic guidance. J Vasc Interv Radiol 2006;17:1511-8. [Crossref] [PubMed]
- Chan JWY, Lau RWH, Chu CM, et al. Expanding the scope of electromagnetic navigation bronchoscopy-guided transbronchial biopsy and ablation with mobile 3D C-arm Machine Cios Spin(®)-feasibility and challenges. Transl Lung Cancer Res 2021;10:4043-6. [Crossref] [PubMed]
- Bhadra K, Rickman OB, Mahajan AK, et al. "Tool-in-lesion" Accuracy of Galaxy System-A Robotic Electromagnetic Navigation BroncHoscopy With Integrated Tool-in-lesion-Tomosynthesis Technology: The MATCH Study. J Bronchology Interv Pulmonol 2023; Epub ahead of print. [Crossref] [PubMed]
- Salahuddin M, Sarkiss M, Sagar AS, et al. Ventilatory Strategy to Prevent Atelectasis During Bronchoscopy Under General Anesthesia: A Multicenter Randomized Controlled Trial (Ventilatory Strategy to Prevent Atelectasis -VESPA- Trial). Chest 2022;162:1393-401. [Crossref] [PubMed]
Cite this article as: Caruso CR, Ma KC, DiBardino DM. Integration of robotic bronchoscopy and cone beam computed tomography: a narrative review. AME Med J 2023;8:34.

