
Management strategies for recurrent pleural effusion: a clinical practice review
Introduction
Pleural effusion represents a significant public health burden in the United States with 1.5 million effusions diagnosed (1) and 170,000 thoracentesis procedures performed (2) annually. A review of the Nationwide Inpatient Sample (NIS) in 2012 identified 126,000 admissions for malignant pleural effusion (MPE) alone (3). Recurrence rates of pleural effusion have been variably reported, but a review of the Surveillance, Epidemiology, and End Results (SEER)-Medicare database for MPE reveals that around half of patients experience recurrence of whom around half recur within two weeks of drainage requiring additional procedures (4). Management of pleural effusions involves a combination of treatment of the underlying cause and interventions to eliminate the effusion and reduce any recurrence, particularly if symptomatic. Definitive management with an indwelling pleural catheter (IPC) with or without pleurodesis is recommended over repeated thoracentesis for recurrent MPE (5,6) and similar options have been extrapolated for the management of recurrent non-malignant pleural effusion (NMPE).
Generally, definitive management of a recurrent pleural effusion involves placement of an IPC, an intervention to achieve pleurodesis, or a combination of the two. Pleurodesis involves the induction of pleural inflammation via chemical agents or mechanically during a video-assisted thoracoscopic procedure, leading to fibrosis and ultimately, obliteration of the pleural space (7). Patients with an IPC may also achieve spontaneous pleurodesis, perhaps through inflammation of the pleura induced by the catheter itself (8).
While much of the available literature on managing recurrent pleural effusion has focused on malignant effusions, there is increasing interest in offering definitive interventions for benign effusions as well. This clinical practice review discusses the different strategies for management of both malignant and NMPEs.
MPE
Development of an MPE is typically indicative of advanced underlying malignancy, therefore management is focused on palliation of symptoms. Definitive treatment emphasizes patient comfort and durability in preventing recurrence or need for repeat intervention. Several studies have evaluated the use of IPC as well as different pleurodesis techniques including both chemical and mechanical. These studies are summarized in Table 1.
Table 1
| Study | Groups | Primary outcomes | Other measures | Adverse events |
|---|---|---|---|---|
| Putnam et al. (9) | Randomized (2:1) | Hospital LOS lower with IPC (1 vs. 6.5 days) | Recurrence of effusion: | 13% complication rate in IPC group. 7% cellulitis and 1% pleural infection |
| IPC (n=96) vs. doxycycline pleurodesis via chest-tube (n=48) | No difference in dyspnea and quality of life | 13% (IPC) vs. 21% (doxycycline) (P=0.446) | ||
| 46% pleurodesis in IPC group at median 26 days | ||||
| Putnam et al. (10) | Retrospective study | Hospital LOS lower with IPC (0 vs. 7.0 days) | Cost benefit when IPC placed as an outpatient vs. inpatient IPC and pleurodesis via chest tube (P<0.001) | 19% overall complication rate in IPC group |
| IPC (outpatient + inpatient) (n=100) vs. doxycycline pleurodesis via chest-tube | No difference in symptom improvement or dyspnea | 20% pleurodesis rate in IPC group | 5% pleural space infection in IPC group requiring antibiotics and further interventions | |
| Hunt et al. (11) | Retrospective study | Overall, LOS lower with IPC (1 vs. 4 days, P=0.006) | Re-intervention rate higher in talc group; 16% vs. 2% (P=0.01) | No difference in complication or mortality |
| IPC (n=54) vs. talc poudrage via thoracoscopy (n=50) | ||||
| Davies et al. (12), TIME-2 Trial | Randomized (1:1) (n=106) | Higher improvement in VAS—dyspnea score in IPC group at 6 months (P=0.01) but not at 6 weeks | Re-intervention rate higher in talc group; 22% vs. 6% (P=0.03) | Overall complication rate higher in the IPC group vs. talc. 40% vs. 12% (P=0.002) (catheter blockage and cellulitis were the commonest) |
| IPC vs. talc slurry via chest tube | LOS shorter in IPC group (0 vs. 4 days) | Spontaneous pleurodesis rate of 51% in IPC group | No difference in serious adverse events between two groups | |
| No difference in quality of life | ||||
| Demmy et al. (13), CALGB 30102 | Randomized (1:1) (n=57) | No difference in lung re-expansion, effusion control or pleurodesis rate between group | Time to removal of catheter was quicker in talc group. Mean of 5 vs. 49 days (P<0.00001) | No major differences reported |
| IPC vs. talc slurry via chest tube | ||||
| Srour et al. (14) | Retrospective study | Pleural effusion control rate higher in IPC vs. talc slurry (52% vs. 34%, P<0.01) | Effusion free survival higher with IPC alone group (101 vs. 58 days, P=0.025) | No differences |
| IPC (n=193) vs. talc slurry via chest tube (n=167) | Days of freedom from catheter not different | |||
| Thomas et al. (15), AMPLE Trial | Randomized (1:1) (n=146) | Hospital LOS (12 months) lower in IPC group vs. talc (10 vs. 12 days, P=0.03) | Re-intervention rate high in talc group vs. IPC (22% vs. 4%, P=0.01) | Overall adverse events higher in IPC (30%) vs. talc (18%) |
| IPC vs. talc slurry via chest tube | No difference in dyspnea-VAS scoring at 12 months | Serious adverse events—1% (IPC) vs. 4% (talc) | ||
| Boshuizen et al. (16) | Randomized (1:1) (n=94) | Hospital LOS lower with IPC group at randomization and total hospitalizations (P<0.05) | Number of re-intervention rate lower with IPC group. (15% vs. 35%, P=0.09) | No difference in adverse events |
| IPC vs. talc slurry via chest tube | ||||
| Bhatnagar et al. (17), TAPPS Trial | Randomized (1:1) (n=330) | No difference in pleurodesis failure rate between groups (22% vs. 24%, P=0.74) | No significant difference in health-related quality of life, chest pain or dyspnea | More cases of pleural infection in poudrage group vs. slurry group (6 vs. 0) |
| Talc poudrage via thoracoscopy vs. talc slurry via chest tube |
IPC, indwelling pleural catheter; LOS, length of stay; VAS, visual analog scale.
IPC and chemical pleurodesis
IPCs emerged as an alternative to pleurodesis for recurrent effusions in the 1990s. Following a series of case reports, a randomized trial evaluated the effectiveness of IPC vs. pleurodesis using doxycycline administered via chest tube in patients with recurrent MPE (9). Spontaneous pleurodesis occurred in 46% of those who received IPC at a median of 29 days; however, accounting for effusion recurrence, sustained pleurodesis occurred in 33% of subjects. The sustained pleurodesis rate with doxycycline was 54%. Similar improvement in subjective scores of dyspnea was observed in both groups at up to 90 days. Patients randomized to IPC spent a median of 1 day in the hospital compared to 6.5 days for those undergoing doxycycline pleurodesis. Subsequent studies have also demonstrated similar findings, namely similar improvement in dyspnea scores between IPC and chemical pleurodesis, with longer inpatient stays among those undergoing pleurodesis (12,15).
The TIME-2 trial randomized patients with recurrent MPE to IPC placement vs. chest tube placement with talc slurry pleurodesis (12). Patients randomized to the IPC group drained the effusion either three times a week or performed symptom-based drainage. There were no significant differences in dyspnea or chest pain scores between groups. Over the course of 1 year of follow-up, 51% of patients with IPC achieved spontaneous pleurodesis and 22% of patients who underwent talc pleurodesis required repeat pleural intervention, leaving a pleurodesis success rate of 78%. Similarly, the AMPLE trial was a study of patients with MPE who were randomized to IPC placement or pleurodesis with talc slurry via chest tube (15). The investigators found that patients receiving IPC had fewer hospital days with similar improvement in dyspnea scores up to 12 months. Talc pleurodesis was successful in 77% of patients, and among those who received IPC, ultimately 29% were able to have the catheter removed after spontaneous pleurodesis.
An important consideration with IPC placement is infectious complications, as these are indwelling foreign bodies that will remain in place for several weeks to months. Infections can include both pleural space and local at the site of catheter insertion. In TIME-2, 13% of patients who received an IPC experienced a pleural infection, a majority of which were serious (requiring intravenous antibiotics), and 12% of patients experienced cellulitis, mostly determined to be nonserious. Other studies, however, have demonstrated lower rates of both pleural infection (1–3%) and cellulitis (4–7%) (9,15,18).
In practice, our approach to the decision of IPC placement vs. chemical pleurodesis is to elicit our patient’s goals for the intervention and pursue shared decision making as both interventions have demonstrated equivalent improvements in dyspnea and quality of life scores. Placing an IPC has the advantage of avoiding an inpatient stay, which is important for many patients who are receiving what is ultimately a palliative intervention and intending to maximize their time at home. However, some patients express hesitancy about their ability to drain a catheter at home, may not be physically able to do so, or have limited social support. For these patients, an attempt at pleurodesis may provide better quality of life. For pleurodesis to be successful there must be apposition of the visceral and parietal pleura, so patients with significant non-expandible lung are not candidates for chemical pleurodesis and should be palliated with an IPC.
IPC-Plus had a novel study design, enrolling patients who received an IPC and at 10 days following placement confirming the absence of non-expandible lung (18). Subjects were then randomized to either talc slurry through the IPC or a placebo instillation. The advantage of instilling through the IPC was to avoid inpatient stay which was a notable disadvantage of chest tube slurry as discussed above. The primary outcome of pleurodesis at 35 days was higher in the talc group (43%) compared to the placebo group (23%), and rates were slightly higher at the full 70 days of follow-up (51% vs. 27%). Notably this pleurodesis rate with talc slurry was significantly lower than that reported in prior studies detailed above, despite a similar rate of spontaneous pleurodesis with placebo. An advantage of the IPC-Plus design was that patients were able to be discharged after talc instillation compared to hospital admission, as occurs with talc slurry administration through a standard non-tunneled chest tube. Patients were drained maximally before talc or placebo instillation, and their next drainage occurred 12–36 hours afterwards and drainage frequency was determined by treating clinicians with a minimum of twice weekly. In our opinion, for effective pleurodesis, a continued apposition of visceral and parietal pleurae is required along with a dry pleural space. However, in this above study, it is likely that fluid reaccumulation, which is also enhanced by the administration of talc and its resultant inflammatory response, largely accounted for the low pleurodesis rate. Hence, if this protocol is employed, we recommend keeping the pleural space as dry as possible with daily drainage post-talc administration to help with pleurodesis.
No further studies using a similar protocol have been published in peer-reviewed literature to our knowledge. However, a conference abstract reviewed a group’s experience with a protocol that involved IPC placement, complete fluid evacuation and confirmation of the absence of non-expandable lung, followed by the administration of a talc slurry (19). Of note, the authors specified that patients drain catheters daily at home. They reported 74% pleurodesis success rate at a median of 20 days, a substantially higher rate compared with IPC-Plus and comparable to rates seen in standard chest tube trials. It is not our typical practice to perform pleurodesis as an outpatient through IPC, though we similarly ask patients to drain their catheter daily to keep the pleural space dry when we employ this approach.
IPC drainage frequency
AMPLE-2 was a study of patients with symptomatic MPE who underwent IPC placement and were randomized to drain the catheter either daily or when symptomatic (20). While there was no difference between groups in breathlessness scores, spontaneous pleurodesis rates were higher in the daily group compared to symptom-guided group at both 60 days (37% vs. 11%) and at 6 months (44% vs. 16%) (P<0.01). Quality of life measures were also better with daily drainage than with symptomatic drainage. The ASAP trial also evaluated alternate day drainage (up to 1 L) with daily drainage (up to 1 L) of IPC with a primary outcome of spontaneous pleurodesis 12 weeks after catheter insertion (21). At 12 weeks pleurodesis was achieved in 47% of those undergoing daily drainage and 24% of those draining on alternate days (P=0.003). Hence, in our practice, we advise patients to drain catheters daily until drainage output slows to less than 150 cc, at which point they may begin draining on alternate days. If they were to drain more than 150 cc consistently on alternate day regimen then we advise to revert to daily drainage again.
Factors affecting pleurodesis
Pleural fluid characteristics
Pleural fluid with low pH (<7.3) and low glucose (<60 mg/dL) has been associated with pleurodesis failure in observational studies, with pH being the strongest predictor with a dose-response relationship of lower pH correlating with greater likelihood of pleurodesis failure (22,23). Fluid pH <7.15 has been associated with >90% likelihood of failure of pleurodesis. In practice, we do not look at pleural fluid studies in isolation but as an additional factor in considering which patients may benefit most from attempted pleurodesis.
Choice of chemical sclerosant for pleurodesis
talc and doxycycline are typical agents used for chemical pleurodesis, with far greater use of talc in the contemporary setting. Other historic agents such as bleomycin, tetracycline, tranexamic acid, Corynebacterium parvum, are not typically used due to greater experience with talc and its superior pleurodesis rates (24,25). These agents have been reviewed in excellent detail elsewhere (26). Kuzdzał et al. studied thoracoscopic administration of a doxycycline solution (with up to 2 additional doses via chest tube if drainage continued to exceed 150 mL/24 h) vs. talc insufflation in patient with MPE (27). Patients were followed for up to 12 months afterwards for pleurodesis success. The trial was ultimately terminated early for significantly greater efficacy in the talc group (100% achieving pleurodesis not requiring re-intervention) compared to doxycycline (46%). Talc is the agent of choice in our practice for chemical pleurodesis.
Route of talc administration
Talc may be administered either as a slurry through a chest tube, as in the above studies comparing efficacy to IPC, or insufflated directly onto the parietal pleural during a medical thoracoscopy (talc poudrage). These have been studied directly in three randomized trials (17,28,29). The two older studies performed thoracoscopy under general anesthesia, and the more recent study performed under moderate sedation. In all studies there was no difference in pleurodesis outcomes and no consistent differences in patient reported outcomes or duration of hospital stay when reported. Our institutional preference is to perform pleurodesis with careful thoracoscopic talc poudrage insufflation, as thoracoscopy with direct visualization ensures the pleural space has been completely dried (to allow good apposition of visceral and parietal pleura) and talc is applied appropriately to the affected areas of parietal pleura. Occasionally patients require thoracoscopy for pleural biopsy in which case talc insufflation can be performed in the same procedure if indicated. It is worth considering that patients who had pleurodesis performed along with placement of the IPC catheter tend to have a shorter “time to removal” of the catheter sometimes a few days to few weeks compared to months with IPC alone. This is important when we consider that local infections as a result of IPC catheter were common at a later time, i.e., after a few weeks of catheter placement and hence “time to catheter removal” amongst these different approaches might play a role.
Future studies and outstanding questions
The AMPLE-3 trial is currently investigating video-assisted thoracoscopic surgery (VATS) guided mechanical pleurodesis compared to IPC alone (30). Other surgical interventions such as pleuro-peritoneal shunting or pleurectomy have shown good efficacy in controlling MPE in observational cohorts, though these have not been studied directly to less invasive therapies and their role in the era of widespread use of IPCs is unclear (31,32).
A recent study evaluated a silver nitrate-coated IPC to a standard uncoated IPC and found no difference in pleurodesis rates (33). Further studies will need to evaluate the role of integrated devices as there is increasing interest in attempts to achieve pleurodesis through an IPC. There is also interest in exploring the role of intrapleural chemotherapy (34,35).
Overall, management of a recurrent MPE requires an interdisciplinary approach taking into consideration the status of the underlying malignancy, planned therapeutics, and patient preferences. IPCs offer likely equivalent degrees of symptom control compared to upfront pleurodesis and are associated with fewer days in the hospital. There is a small risk of infection, most commonly cellulitis at the insertion site, or less commonly a pleural infection. To maximize rates of spontaneous pleurodesis and symptom improvement, patients should be advised to drain catheters daily, if possible, rather than as needed based on symptoms. Patients who do not wish to have a catheter remaining in place for a prolonged period or are unable to drain an IPC at home should be considered for pleurodesis with talc as the agent of choice. This may be done either via a standard chest tube with a talc slurry or during a thoracoscopy with talc poudrage insufflation. Chest tube and thoracoscopic pleurodesis have likely equivalent efficacy, with the ability to take pleural biopsies a notable advantage of a thoracoscopic approach. While there is increasing interest in avoiding hospitalization for patients with a limited life expectancy, pleurodesis through an IPC requires further study including the integration of novel devices to enhance likelihood of a successful pleurodesis.
NMPE
Management of NMPE is predominantly aimed at treating the underlying cause. Recurrent transudative effusions are typically secondary to congestive heart failure (CHF), cirrhosis or nephrotic syndrome and these conditions are managed with a combination of diuretics and treatment of the underlying disease. For symptomatic patients who do not respond to medical management, a more definitive intervention may be considered. Like MPE, definitive management options include IPC placement or chemical pleurodesis, again usually with talc. Studies evaluating these approaches have been summarized in Table 2.
Table 2
| Study | Groups | Primary outcomes | Other measures | Adverse events |
|---|---|---|---|---|
| Freeman et al. (36) | Retrospective study | Hospital LOS higher with talc group (6 vs. 2 days, P<0.0001) | 35% spontaneous pleurodesis rate in IPC group at mean of 5 months | Only one death occurred in the whole cohort in the talc group—attributed to talc-induced respiratory failure |
| IPC (n=40) vs. talc poudrage via thoracoscopy (n=40) | Readmission rate higher with talc group (23% vs. 5%, P=0.048) | |||
| Majid et al. (37) | Retrospective study | Pleurodesis success higher in group 1 (80% vs. 25%, P<0.05) | Overall pleurodesis rate of 44% | No significant differences in adverse events reported between two groups |
| Talc poudrage via thoracoscopy with IPC (group 1) vs. IPC alone (group 2) | Median time to catheter removal was quicker in group 1 (11 vs. 66 days) | Re-intervention rate and hospital re-admissions lower in group 1 (P<0.05) | ||
| Walker et al. (38) | Randomized study | No significant difference in mean breathless score between groups | No difference in mean number of beds days, pleurodesis success | Overall adverse events higher in IPC group—59% vs. 37% (P=0.04) |
| IPC (n=33) vs. TT (n=35) |
IPC, indwelling pleural catheter; LOS, length of stay; TT, therapeutic thoracentesis.
Another important distinction is that development of MPE is a sign of advanced malignancy and often a poor underlying prognosis. Patients may therefore consider interventions that prioritize avoiding inpatient stays, as is typically the case when pursuing pleurodesis. On the other hand, an indwelling catheter may be anticipated to remain in place for longer if placed for a benign effusion, which may impact complications or patient satisfaction with draining at home. Also, unlike MPE, there is a paucity of high-quality data to guide treatment decision making, with most data coming from retrospective reviews.
The only published randomized trial (the REDUCE study) at time of this publication enrolled patients with recurrent effusion from CHF, liver failure, or renal disease (38). Subjects were randomized to IPC drained at least three times weekly at home or medical management with an initial thoracentesis and subsequent thoracentesis as needed based on symptoms. Of note, the study did not achieve the planned enrollment within the time period. There was no difference in dyspnea score between groups averaged across the 12-week follow-up period. Review of visual dyspnea score over time seemed to show some separation of scores in favor of IPC in the latter half of the follow-up period, however this was non-significant. There were no differences in outcomes when looking just at those with heart failure/renal failure or liver failure. The spontaneous pleurodesis rate with IPC placement in this study was 13%, far lower than reported in studies of MPE as above. Patients randomized to thoracentesis received on average 2–3 thoracentesis over the study period, and 17% ultimately required chest tube, IPC, or thoracoscopy. There were overall more adverse events in the IPC group, typically pain, infection (cellulitis or pleural), and fluid leakage. There were no differences in rates of underlying disease decompensation, however. The remainder of studies in transudative NMPE are observational in nature and will be organized by underlying etiology along with summarized recommendations.
CHF
Tunneled Pleural Catheters for Refractory Effusions Attributed to Congestive Heart Failure (TREAT-CHF) was an attempted randomized trial of IPC vs. medical therapy in patients with recurrent symptomatic pleural effusion due to CHF (39). Unfortunately, this study appears to have been withdrawn due to poor enrollment. Observational studies of IPC in CHF suggest improvement in dyspnea scores following IPC placement and reduction in admissions for CHF (14,37). Spontaneous pleurodesis rate with IPC alone across multiple studies ranged from 24–35% and occurred in 2 studies at a median of 66 days and in another at a mean of 5 months, though with considerable range of around 1–8 months (14,36,37,40). Infectious complications were not reported with IPC in one study (14). Other studies noted catheter associated infections in 3/28 patients (11%) (including one case of cellulitis and two of pleural infection) (37) and 1/40 patients (2.5%, cellulitis) (36). These complication rates are overall comparable to rates found with IPC placement for MPE.
Two studies have also retrospectively reviewed outcomes after thoracoscopy with talc pleurodesis for CHF. Majid and colleagues reported a pleurodesis rate of 80% occurring at a median of 12 days (range, 2–22 days) compared to 25% in patients with IPC alone (37). Notably, patients in this study had IPC placed in addition to chest tube, which remained in place to continue drainage as outpatient. In another study, Freeman and colleagues did not specifically report pleurodesis rates, but only 5% of patients who underwent thoracoscopic pleurodesis required an additional pleural drainage procedure (36). Complication rates with talc were high in this study—23% requiring readmission, 12.5% with respiratory failure, and 5% operative mortality rate. In contrast, Majid et al. reported only 1 case (out of 13) with postoperative complication that was periprocedural hypotension related to anesthesia.
Overall, limited data suggests IPC placement may improve symptoms in patients with recurrent pleural effusion refractory to medical therapy in patients with CHF. Spontaneous pleurodesis rates and infectious complications are comparable to those reported for MPE. Talc pleurodesis via medical thoracoscopy is feasible in achieving pleurodesis in this population but may carry a high risk of perioperative complications. We favor a multidisciplinary approach to managing these patients in close coordination with our cardiology/advanced heart failure teams. If patients are significantly dyspneic from recurrent effusions, improve following thoracentesis, and are refractory to medical therapy we consider IPC placement and ideally talc poudrage via thoracoscopy if possible.
Hepatic hydrothorax (HH)
Management of HH that is refractory to a low-salt diet and diuretics differs from other causes of transudative effusions in that there exists a definitive treatment, liver transplantation. Presence of HH did not portend a poorer prognosis after liver transplant in a review of 28 patients with HH and 56 controls, and by 3 months postoperatively only one patient had a persistent effusion that was suspected to be secondary to CHF (41). For patients in whom liver transplant is not an option or in whom an intervention is needed prior to transplant, transjugular intrahepatic portosystemic shunting (TIPS) is a reasonable consideration. HH likely accumulates secondary to ascitic fluid tracking across the diaphragm, and TIPS has been shown to be effective in management of refractory ascites in a randomized trial (42). Across several observational studies, overall response rate of HH to TIPS has ranged from 58–82% (43). Not all patients may be candidates for TIPS, as bypassing portal circulation can exacerbate complications of synthetic dysfunction, in particular hepatic encephalopathy. Case reports and series have documented success with VATS for closure of diaphragmatic defects in carefully selected patients, with a major determinant of success seemingly whether or not clear diaphragmatic defects are able to be identified intraoperatively (44-46).
IPC placement has been retrospectively reviewed for HH. Reported pleurodesis rates range from 15–28% across studies (40,47,48), occurring in the largest available series at a median of 55 days (47). Infectious complications are of a potentially greater concern in patients with HH given impaired immunity secondary to their underlying cirrhosis and often poorer nutritional status that also impacts wound healing. Pleural infections in the two largest series identified occurred in 10–16% of patients and associated mortality occurred in 25–30% of these cases (47,48). Skin infections occurred in 2–5% of cases in these series.
Renal complications are another feared complication of ongoing drainage of pleural fluid in patients with HH. The massive ongoing loss of fluid and albumin following chest tube placement has been associated with development of renal injury in up to almost two-thirds of patients such that chest tube placement is strongly discouraged for HH (49,50). A retrospective study of IPC placement that commented upon renal complications noted 2/79 cases (2.5%) with renal failure and 1 case of a severe electrolyte disturbance secondary to the catheter (47).
A meta-analysis of case series documenting pleurodesis rates for HH found a pooled success rate of 72%, with similar rates for medical thoracoscopy and VATS approach and across chemical agents used (51). The pooled complication rate was 82% across the series that reported this data, particularly renal failure in 18% and infectious complications including pneumonia (10%) and empyema (6%). Of note postoperative liver failure occurred in 10% of patients as well. Thus, while pleurodesis was overall successful in most patients across case series the complication rate was high. Our own practice is generally to not place IPC or pursue pleurodesis for patients with recurrent HH given the high associated risk of complications, unless the patient is pursuing a comfort-care/hospice-care treatment strategy. In the latter case, the risks/benefits are discussed with the patient and family in detail. Those wishing to continue aggressive medical therapy but who are not currently candidates for a more definitive intervention such as transplant or TIPS are typically palliated with as-needed thoracentesis.
Chylothorax
Chylothorax represents a unique non-MPE secondary to buildup of chyle in the pleural cavity from either traumatic or non-traumatic insults. Traumatic causes include cardiothoracic surgery or trauma (blunt or penetrating) to the thoracic duct. Non-traumatic causes included malignancy, most commonly lymphoma, CHF, cirrhosis, and others (52). Management of chylothorax depends not only on the etiology but also the degree of reaccumulation. There are unfortunately no prospective or randomized studies so management is guided by retrospective data and expert consensus. Conservative measures that should be considered initially in all patients (in addition to drainage of fluid for symptomatic relief) include dietary modification and medical therapy. Dietary change to a low-fat diet focused on eliminating long chain triglycerides may help slow/eliminate reaccumulation as these are taken up by the lymphatic system in chylomicrons and promote flow of chyle in the thoracic duct. With dietary measures and drainage, resolution has been reported in 28–79% of traumatic and 44–87% of nontraumatic cases (53). Medical therapy includes somatostatin or its synthetic analogue octreotide, which in conjunction with dietary changes has been found in a small series of seven patients with malignancy to lead to resolution of chylothorax (54) and in review of post-cardiac surgery chylothorax to lead to resolution in ~90% of cases (55). For patients refractory to conservative therapy or with large volume output a definitive intervention needs to be considered. These include primarily surgical thoracic duct ligation or lymphangiography with thoracic duct embolization by interventional radiology (IR). A surgical approach has the advantage of a higher success rate [~80–90% (53)] at the cost of a more invasive intervention compared to an IR approach, which is successful in ~60% of cases (56). For both techniques, reported success rates are higher for traumatic than for non-traumatic cases.
IPCs are also occasionally placed for persistent chylothorax. Jimenez and colleagues retrospectively reviewed 19 patients with recurrent symptomatic chylothorax secondary to malignancy, among whom 10 were managed with IPC (57). Spontaneous pleurodesis or chylothorax resolution with IPC occurred in 55% at a median of 26 days with only one patient experiencing complication of a clogged catheter. Subsequently, DePew and colleagues retrospectively reviewed a series of 11 patients (14 hemithoraces) with benign chylothorax (both traumatic n=3 and non-traumatic n=8) managed with IPC (58). Non-traumatic etiologies included yellow nail syndrome, lymphangioleiomyomatosis, chylous ascites, and idiopathic. They found that 64% achieved pleurodesis, at a median time of 176 days. Ultimately, pleurodesis success rate was similar to the series of patients with malignancy but at a much longer median time to removal, likely reflecting the lack of a readily treatable underlying cause. Three patients experienced an occlusion of their IPC. In both series there were no major nutritional complications, an important theoretical consideration with draining large amount of chyle. Talc pleurodesis during medical thoracoscopy has also been retrospectively reviewed for malignant chylothorax secondary to lymphoma, with 100% successful rate of pleurodesis in patients surviving to up to 90 days post-intervention (59). Our approach in treating these patients is highly individualized based on the etiology of chylothorax, amount of output, and degree of symptomatology. This typically involves discussions with our colleagues in surgery, IR, and oncology, and an assessment of patient preferences and candidacy for invasive interventions.
Strengths and limitations of this review
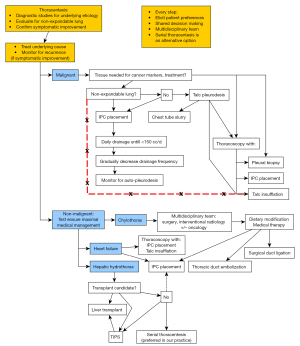
Herein we present a review of management strategies for recurrent pleural effusions of malignant and non-malignant etiologies. The review is organized around answering relevant clinical questions, and recommendations based on the evidence presented and our own clinical experience and practice are provided throughout and summarized in the end (Table 3). Further, we have synthesized an overall algorithm of management to provide a readily accessible aid to clinicians managing such patients (Figure 1).
Table 3
| Clinical scenario | Recommendation |
|---|---|
| Malignant effusions | |
| IPC vs. chemical pleurodesis | Recommend shared decision making given roughly equivalent outcomes but specific pros and cons with each approach |
| Frequency of IPC drainage | Daily drainage provides better palliation and faster spontaneous pleurodesis |
| Choice of chemical sclerosant | Talc is the agent of choice for chemical pleurodesis |
| Route of talc administration | Equivalent outcomes with chest tube slurry vs. thoracoscopic poudrage. Our institutional practice is to prefer poudrage via medical thoracoscopy |
| Non-malignant effusions | |
| CHF | Consider IPC or pleurodesis for symptomatic effusions with improvement following thoracentesis and refractory to medical therapy |
| Hepatic hydrothorax | If not a candidate for transplant or TIPS, we typically perform serial thoracentesis given high risk for complications with IPC or pleurodesis |
| Chylothorax | Best management with dietary modification, medical therapy, and a multidisciplinary approach for definitive intervention |
IPC, indwelling pleural catheter; CHF, congestive heart failure; TIPS, transjugular intrahepatic portosystemic shunting.
Although a comprehensive literature search was undertaken and we have attempted to organize evidence from the most relevant primary literature throughout, this was not a systematic review. Further, no quantitative meta-analysis was performed nor was a formal assessment for publication bias in this review. Nonetheless, we believe this pragmatic review provides clinicians a resource that highlights both the underlying primary literature and clinical expertise to inform optimal care for patients with recurrent pleural effusions.
Conclusions
Definitive management strategies for recurrent pleural effusions can improve symptoms and quality of life for patients as well as reduce unnecessary repeated procedures and hospitalizations. Techniques including the placement of an IPC as well as thoracoscopic and chemical pleurodesis have been described in the literature and can be offered to patients depending on the specific clinical scenario. Most importantly, it is critical to carefully assess the etiology of the pleural effusion and approach each patient with a strategy that is consistent with their goals, preferences, and best interest.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathan Kurman and Bryan S. Benn) for the series “Diagnostic & Therapeutic Bronchoscopy” published in AME Medical Journal. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-107/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-107/coif). The series “Diagnostic & Therapeutic Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Light RW. Pleural Diseases. 5th Edition. Baltimore: Lippincott Williams & Wilkins; 2007.
- Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13 1998;1-119. [PubMed]
- Taghizadeh N, Fortin M, Tremblay A. US Hospitalizations for Malignant Pleural Effusions: Data From the 2012 National Inpatient Sample. Chest 2017;151:845-54. [Crossref] [PubMed]
- Ost DE, Niu J, Zhao H, et al. Quality Gaps and Comparative Effectiveness of Management Strategies for Recurrent Malignant Pleural Effusions. Chest 2018;153:438-52. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii32-40. [Crossref] [PubMed]
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:839-49. [Crossref] [PubMed]
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012;83:91-8. [Crossref] [PubMed]
- Sørensen PG, Svendsen TL, Enk B. Treatment of malignant pleural effusion with drainage, with and without instillation of talc. Eur J Respir Dis 1984;65:131-5. [PubMed]
- Putnam JB Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992-9. [Crossref] [PubMed]
- Putnam JB Jr, Walsh GL, Swisher SG, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg 2000;69:369-75. [Crossref] [PubMed]
- Hunt BM, Farivar AS, Vallières E, et al. Thoracoscopic talc versus tunneled pleural catheters for palliation of malignant pleural effusions. Ann Thorac Surg 2012;94:1053-7; discussion 1057-9. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Demmy TL, Gu L, Burkhalter JE, et al. Optimal management of malignant pleural effusions (results of CALGB 30102). J Natl Compr Canc Netw 2012;10:975-82. [Crossref] [PubMed]
- Srour N, Potechin R, Amjadi K. Use of indwelling pleural catheters for cardiogenic pleural effusions. Chest 2013;144:1603-8. [Crossref] [PubMed]
- Thomas R, Fysh ETH, Smith NA, et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA 2017;318:1903-12. [Crossref] [PubMed]
- Boshuizen RC, Vd Noort V, Burgers JA, et al. A randomized controlled trial comparing indwelling pleural catheters with talc pleurodesis (NVALT-14). Lung Cancer 2017;108:9-14. [Crossref] [PubMed]
- Bhatnagar R, Piotrowska HEG, Laskawiec-Szkonter M, et al. Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients With Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2020;323:60-9. [Crossref] [PubMed]
- Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient Talc Administration by Indwelling Pleural Catheter for Malignant Effusion. N Engl J Med 2018;378:1313-22. [Crossref] [PubMed]
- Fitzgerald DB, Muruganandan S, Stanley C, et al. EPIToME (Early Pleurodesis via IPC with Talc for Malignant Effusion): Evaluation of a new management algorithm. Eur Respir J 2019;54:OA493.
- Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med 2018;6:671-80. [Crossref] [PubMed]
- Wahidi MM, Reddy C, Yarmus L, et al. Randomized Trial of Pleural Fluid Drainage Frequency in Patients with Malignant Pleural Effusions. The ASAP Trial. Am J Respir Crit Care Med 2017;195:1050-7. [Crossref] [PubMed]
- Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest 2000;117:87-95. [Crossref] [PubMed]
- Rodríguez-Panadero F, López Mejías J. Low glucose and pH levels in malignant pleural effusions. Diagnostic significance and prognostic value in respect to pleurodesis. Am Rev Respir Dis 1989;139:663-7. [Crossref] [PubMed]
- Sahn SA. Talc should be used for pleurodesis. Am J Respir Crit Care Med 2000;162:2023-4; discussion 2026. [Crossref] [PubMed]
- Mohamed E, Ali E, Mahmoud H. Pleurodesis for malignant pleural effusions: A comparison of bleomycin or tranexamic acid alone versus a combination of both. Eur Respir J 2013;42:3072.
- Dipper A, Jones HE, Bhatnagar R, et al. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2020;4:CD010529. [Crossref] [PubMed]
- Kuzdzał J, Sładek K, Wasowski D, et al. Talc powder vs doxycycline in the control of malignant pleural effusion: a prospective, randomized trial. Med Sci Monit 2003;9:PI54-9. [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [Crossref] [PubMed]
- Yim AP, Chan AT, Lee TW, et al. Thoracoscopic talc insufflation versus talc slurry for symptomatic malignant pleural effusion. Ann Thorac Surg 1996;62:1655-8. [Crossref] [PubMed]
- Fitzgerald DB, Sidhu C, Budgeon C, et al. Australasian Malignant PLeural Effusion (AMPLE)-3 trial: study protocol for a multi-centre randomised study comparing indwelling pleural catheter (±talc pleurodesis) versus video-assisted thoracoscopic surgery for management of malignant pleural effusion. Trials 2022;23:530. [Crossref] [PubMed]
- Kara M, Alzafer S, Okur E, et al. The use of single incision thoracoscopic pleurectomy in the management of malignant pleural effusion. Acta Chir Belg 2013;113:270-4. [Crossref] [PubMed]
- Genc O, Petrou M, Ladas G, et al. The long-term morbidity of pleuroperitoneal shunts in the management of recurrent malignant effusions. Eur J Cardiothorac Surg 2000;18:143-6. [Crossref] [PubMed]
- Shrager JB, Bhatnagar R, Kearney CT, et al. Silver Nitrate-coated versus Standard Indwelling Pleural Catheter for Malignant Effusions: The SWIFT Randomized Trial. Ann Am Thorac Soc 2022;19:1722-9. [Crossref] [PubMed]
- Hu R, Jiang H, Li H, et al. Intrapleural perfusion thermo-chemotherapy for pleural effusion caused by lung carcinoma under VATS. J Thorac Dis 2017;9:1317-21. [Crossref] [PubMed]
- Pan P, Wu F, Xu Z, et al. Intrapleural treatment in patients with non-small cell lung cancer with malignant pleural effusions in the real world. Thorac Cancer 2021;12:3416-25. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Dake M, et al. A propensity-matched comparison of pleurodesis or tunneled pleural catheter for heart failure patients with recurrent pleural effusion. Ann Thorac Surg 2014;97:1872-6; discussion 1876-7. [Crossref] [PubMed]
- Majid A, Kheir F, Fashjian M, et al. Tunneled Pleural Catheter Placement with and without Talc Poudrage for Treatment of Pleural Effusions Due to Congestive Heart Failure. Ann Am Thorac Soc 2016;13:212-6. [Crossref] [PubMed]
- Walker SP, Bintcliffe O, Keenan E, et al. Randomised trial of indwelling pleural catheters for refractory transudative pleural effusions. Eur Respir J 2022;59:2101362. [Crossref] [PubMed]
- Oh S. Tunneled Pleural Catheters for Refractory Effusions Attributed to Congestive Heart Failure (TREAT-CHF) Trial ClinicalTrials.gov identifier: NCT03696524 [updated 4/5/2023. Available online: https://www.clinicaltrials.gov/study/NCT03696524
- Frost N, Ruwwe-Glösenkamp C, Raspe M, et al. Indwelling pleural catheters for non-malignant pleural effusions: report on a single centre's 10 years of experience. BMJ Open Respir Res 2020;7:e000501. [Crossref] [PubMed]
- Xiol X, Tremosa G, Castellote J, et al. Liver transplantation in patients with hepatic hydrothorax. Transpl Int 2005;18:672-5. [Crossref] [PubMed]
- Rössle M, Ochs A, Gülberg V, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med 2000;342:1701-7. [Crossref] [PubMed]
- Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut 2010;59:988-1000. [Crossref] [PubMed]
- Huang PM, Chang YL, Yang CY, et al. The morphology of diaphragmatic defects in hepatic hydrothorax: thoracoscopic finding. J Thorac Cardiovasc Surg 2005;130:141-5. [Crossref] [PubMed]
- Temes RT, Davis MS, Follis FM, et al. Videothoracoscopic treatment of hepatic hydrothorax. Ann Thorac Surg 1997;64:1468-9. [Crossref] [PubMed]
- Mouroux J, Perrin C, Venissac N, et al. Management of pleural effusion of cirrhotic origin. Chest 1996;109:1093-6. [Crossref] [PubMed]
- Shojaee S, Rahman N, Haas K, et al. Indwelling Tunneled Pleural Catheters for Refractory Hepatic Hydrothorax in Patients With Cirrhosis: A Multicenter Study. Chest 2019;155:546-53. [Crossref] [PubMed]
- Kniese C, Diab K, Ghabril M, et al. Indwelling Pleural Catheters in Hepatic Hydrothorax: A Single-Center Series of Outcomes and Complications. Chest 2019;155:307-14. [Crossref] [PubMed]
- Orman ES, Lok AS. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int 2009;3:582-6. [Crossref] [PubMed]
- Liu LU, Haddadin HA, Bodian CA, et al. Outcome analysis of cirrhotic patients undergoing chest tube placement. Chest 2004;126:142-8. [Crossref] [PubMed]
- Hou F, Qi X, Guo X. Effectiveness and Safety of Pleurodesis for Hepatic Hydrothorax: A Systematic Review and Meta-Analysis. Dig Dis Sci 2016;61:3321-34. [Crossref] [PubMed]
- Agrawal A, Chaddha U, Kaul V, et al. Multidisciplinary Management of Chylothorax. Chest 2022;162:1402-12. [Crossref] [PubMed]
- Bender B, Murthy V, Chamberlain RS. The changing management of chylothorax in the modern era. Eur J Cardiothorac Surg 2016;49:18-24. [Crossref] [PubMed]
- Mincher L, Evans J, Jenner MW, et al. The successful treatment of chylous effusions in malignant disease with octreotide. Clin Oncol (R Coll Radiol) 2005;17:118-21. [Crossref] [PubMed]
- Ismail NA, Gordon J, Dunning J. The use of octreotide in the treatment of chylothorax following cardiothoracic surgery. Interact Cardiovasc Thorac Surg 2015;20:848-54. [Crossref] [PubMed]
- Kim PH, Tsauo J, Shin JH. Lymphatic Interventions for Chylothorax: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol 2018;29:194-202.e4. [Crossref] [PubMed]
- Jimenez CA, Mhatre AD, Martinez CH, et al. Use of an indwelling pleural catheter for the management of recurrent chylothorax in patients with cancer. Chest 2007;132:1584-90. [Crossref] [PubMed]
- DePew ZS, Iqbal S, Mullon JJ, et al. The role for tunneled indwelling pleural catheters in patients with persistent benign chylothorax. Am J Med Sci 2013;346:349-52. [Crossref] [PubMed]
- Mares DC, Mathur PN. Medical thoracoscopic talc pleurodesis for chylothorax due to lymphoma: a case series. Chest 1998;114:731-5. [Crossref] [PubMed]
Cite this article as: Ajmani G, Ravikumar N, Wagh A. Management strategies for recurrent pleural effusion: a clinical practice review. AME Med J 2023;8:35.

