
Evaluation & management of clinically significant hemoptysis—a clinical practice review
Introduction
Background
Hemoptysis is the expectoration of blood originating from the tracheobronchial tree or pulmonary parenchyma and can vary in presentation from mild, blood-tinged mucous to severe life-threatening hemorrhage (1). The etiologies of hemoptysis vary with the incidence shifting with advances in medical and surgical therapies over the years (2). Hemoptysis can be fatal if not acted on appropriately, with the cause of death typically being from hypoxia leading to asphyxiation and not exsanguination. Unlike hemorrhage in other scenarios, small amounts of bleeding in the airway can lead to clinical instability—especially in individuals with underlying lung disease. Historically, mortality rates were greater than 75% due to few therapeutic options, leading to surgery becoming the mainstay of intervention (3,4).
Patients presenting with massive or life-threatening hemoptysis present a diagnostic and therapeutic challenge to physicians, and require immediate evaluation and multidisciplinary involvement of intensive care physicians, interventional pulmonology, interventional radiology, and occasionally thoracic surgery. Localization of hemorrhage is imperative—with subsequent control and definitive management. Fortunately, advancements in diagnostic and therapeutic options available to clinicians have significantly improved mortality rates in patients presenting with life-threatening hemoptysis.
Rationale and knowledge gap
While mortality has significantly improved over the years with these advancements in diagnostic and therapeutic options, the bulk of existing literature is comprised of single center, retrospective reports with small sample sizes often with heterogenous populations (5). The paucity of rigorous studies to help guide treatment requires frequent review of existing literature to help clinicians provide the best care to individuals presenting with hemoptysis.
Objective
Due to the complex nature of hemoptysis management and lack of guiding evidence with randomized controlled trials, we hope to summarize the available evidence to provide a comprehensive approach to diagnosis and management.
Definitions
The severity of hemoptysis has been variably described, with massive hemoptysis being the descriptor most widely utilized for life threatening bleeding. While there is no universally accepted volume that defines massive hemoptysis, the term is typically used to represent any volume of blood in the airway that is an imminent threat to life (6). Some definitions stratify based on volume of bleeding per hour, while others stratify quantifying the volume of bleeding over 24 hours. Ranges of 200–1,000 mL/24 hours have been described, due to the anatomic dead space of the tracheobronchial tree being approximately 150–200 mL with physicians most commonly defining massive hemoptysis as expectorated blood of 600 mL over 24 hours (2,6,7). While 400 mL of blood in the alveolar space has been observed to impair oxygen transfer (7), one retrospective study of 1,087 patients with massive hemoptysis followed over a 14-year period, had an average volume of expectorated blood of 218 mL (8). Due to the small volume of alveolar dead space, small amounts of bleeding can cause clinically significant complications—especially in those with underlying lung disease.
Approximation of the amount of blood expectorated can be challenging and is often over or under-estimated. More importantly, the clinical significance of hemoptysis on the patient should be used to risk stratify the severity. Others have argued that the definition of massive hemoptysis be replaced with life-threatening hemoptysis if patients develop respiratory failure, airway obstruction, or hypotension (9). Patients with underlying lung disease already have increased physiologic dead space which can create challenges for patients to compensate for even small amounts of bleeding (2,10). Ultimately, any amount of hemoptysis causing respiratory failure from airway obstruction or hypotension should be considered life-threatening hemoptysis (9).
Epidemiology & etiology
Life-threatening hemoptysis is relatively uncommon, with only 5–14% of patients presenting with hemoptysis suffering from life-threatening clinical features (4,11,12). Patients that are at risk for hemoptysis will typically experience a single, non-life-threatening episode that is self-limiting. However, careful attention should be paid to these patients, as this could represent a sentinel bleed that progresses to life-threatening hemoptysis. While 80% of lung cancer patients will never develop hemoptysis, 0.6–3% will develop life-threatening hemoptysis (13-15). A sentinel bleed is common having been reported in 80% of patients who develop life threatening bleeding related to malignancy (16).
Previously, few therapeutic options were available for patients presenting with life threatening hemoptysis with mortality rates between 50–100% (3,4). In the late 1970’s, Garzon and Gourin published outcomes of patients presenting with massive hemoptysis, citing improved mortality to 17% with initiation of early operative intervention; leading to surgery gaining a prominent role for localization and management (4,5). One study of 59 patients presenting with hemoptysis revealed a mortality rate of 59% in patients with malignancy and 58% in patients with bleeding of greater than 1,000 mL over 24 hours, but was less than 10% if the bleeding was less than 1,000 mL over 24 hours (12). With advancements in therapeutic options, mortality rates have decreased to 9–38% (17-20).
Certain risk factors have been found to be associated with an increased risk in mortality. A retrospective cohort study of 1,087 patients presenting with severe hemoptysis developed a mortality risk score and found that chronic alcoholism, pulmonary artery involvement, hemorrhage affecting two or more quadrants on admission chest radiograph, aspergillosis, cancer, and need for mechanical ventilation were independently associated with increased risk for mortality (8). Each risk factor was assigned one to two points with the cumulative score predicting increasing mortality ranging from 2% (1 point) to 91% (7 points).
The causes of hemoptysis are diverse, ranging from malignancy, infectious, and non-infectious (Table 1). In patients presenting with hemoptysis, it is important to rule out mimics such as nasopharyngeal or gastrointestinal bleeding. Worldwide, the most common cause of hemoptysis is tuberculosis, with malignancy being the most common cause in developed countries (11,21-25). A 2018 observational, multicenter review from Europe of 606 patients found that pulmonary malignancy, pneumonia/lung abscess and bronchiectasis were the most frequent causes of hemoptysis (25). Malignancy and bronchiectasis were found to be the leading causes of moderate and severe hemoptysis, with pneumonia and acute bronchitis being the most frequently associated with mild bleeding (25).
Table 1
| System | Disease |
|---|---|
| Cardiovascular | Aortic aneurysm or bronchovascular fistula |
| Arteriovenous malformation | |
| Congenital heart disease | |
| Congestive heart failure | |
| Mitral stenosis | |
| Pulmonary embolism/infarct | |
| Primary pulmonary hypertension | |
| Pulmonary artery aneurysm | |
| Ruptured thoracic aneurysm | |
| Infectious | Bronchitis/pneumonia from bacterial and viral illnesses |
| Lung abscess | |
| Mycetoma/invasive pulmonary fungal disease (aspergillosis) | |
| Necrotizing pneumonia | |
| Parasites | |
| Septic emboli | |
| Tuberculosis/nontuberculous mycobacteria | |
| Neoplastic | Bronchogenic carcinoma |
| Endobronchial tumor | |
| Pulmonary metastases | |
| Sarcoma | |
| Pulmonary | Bronchiectasis |
| Broncholithiasis | |
| Cystic fibrosis | |
| Lymphangioleiomyomatosis | |
| Autoimmune | Anti-glomerular basement membrane disease |
| Anti-phospholipid syndrome | |
| Bechet’s disease | |
| Cryoglobulinemia | |
| Diffuse alveolar hemorrhage from vasculitis | |
| Goodpasture syndrome | |
| Granulomatous with polyangiitis | |
| Henoch-Schonlein purpura | |
| Microscopic polyangiitis | |
| Mixed connective tissue disease | |
| Rheumatoid arthritis | |
| Systemic lupus erythematosus | |
| Systemic sclerosis | |
| Hematologic | Disseminated intravascular coagulation |
| Iatrogenic coagulopathies (anticoagulants/anti-platelet medications) | |
| Platelet disease | |
| Thrombotic thrombocytopenic purpura | |
| Other | Cryptogenic |
| Drugs: anticoagulants/antiplatelet, bevacizumab, crack/cocaine, nitrofurantoin | |
| Foreign body aspiration | |
| Iatrogenic | |
| Trauma |
Hemoptysis from pulmonary malignancy is typically mild in nature and usually a result of the friable tumor developing neo-angiogenesis from systemic circulation (6). Conversely, massive hemoptysis is rare and is typically caused by direct invasion of major vessels with subsequent necrosis of the invading tumor (26). A large retrospective analysis of 877 cases of lung cancer showed that massive hemoptysis was the cause of death in only 3.3% of cancer patients (16). Squamous cell carcinoma is most classically associated with an increased risk of hemoptysis. A prospective study of 125 patients with non-small cell lung cancer who presented with hemoptysis revealed that 52% of the patients had a diagnosis of squamous cell carcinoma with 31% having adenocarcinoma (27). Risk factors associated with hemoptysis were advanced disease and centrally located lesions, with non-small cell lung cancer accounting for 11% of the ICU hemoptysis related admissions.
There are a variety of iatrogenic causes of hemoptysis that can be minimized by careful patient selection for procedures. While minor bleeding can occur from endoscopic trauma during bronchoscopy, significant bleeding is typically associated with diagnostic procedures such as biopsy or therapeutic interventions. A 2017 review found that the incidence of bleeding for diagnostic bronchoscopy ranged from 0.26% to 5% (28). Results from the AQuIRE registry and another multicenter prospective study reveal that traditional diagnostic procedures such as airway inspection, bronchoalveolar lavage and endobronchial ultrasound with transbronchial needle aspiration are safe with low bleeding rates (29,30). Careful patient selection and medication reconciliation are critical for minimizing iatrogenic complications. Low-dose aspirin is typically safe to continue; while clopidogrel, warfarin, direct acting oral anticoagulants (DOACs), heparin, and low-molecular-weight heparin need to be held for transbronchial biopsy and other therapeutic interventions. Aspirin alone has been shown to be safe in patients undergoing transbronchial lung biopsy. A prospective cohort study of 1,217 patients undergoing biopsy revealed that the risk of minor bleeding was not increased in patients on aspirin compared to controls (31). Less than a 2% risk of minor bleeding and less than a 1% risk of severe bleeding was seen in those patients where aspirin was continued. Another prospective cohort study of 604 patients undergoing transbronchial lung biopsy showed excessive increased risk of bleeding in patients where clopidogrel was continued (32). Significant bleeding was seen in 89% (16 of 18 patients) of patients where clopidogrel was continued, versus 3.4% of controls causing the study to be stopped after 6 months. Unsurprisingly, all 12 patients receiving both aspirin and clopidogrel experienced either moderate or severe bleeding.
Thrombocytopenia is another factor that can increase the risk of iatrogenic hemoptysis and should be treated accordingly. A case-series of 24 patients with thrombocytopenia (mean platelet count of 30,000/mm3) undergoing transbronchial lung biopsies revealed 20% of patients suffered a hemorrhagic complication with those suffering from a complication having a mean platelet count of 20,600/mm3 (33). There is a paucity of data on the safe minimum platelet count threshold for patients undergoing transbronchial biopsies, however, 50,000/mm3 has been suggested as an acceptable threshold (33,34).
There are a variety of cases where the etiology of hemoptysis remains unknown. Cryptogenic or idiopathic major hemoptysis is uncommon, with most causes evident with simple investigations like bronchoscopy (6). The incidence of cryptogenic hemoptysis is variably reported in the literature and ranges from 7–25% (6,35,36). Certain angiographic features associated with cryptogenic hemoptysis include small or microaneurysms, extravasation, involvement of bronchial systemic artery, hypervascularity in the contralateral bronchial arteries and/or ipsilateral bronchial artery branches with bronchial artery embolism being highly effective for management (36,37).
Anatomy and pathophysiology
Management of hemoptysis requires an in-depth understanding of pulmonary circulation. The lungs are unique in having a dual blood supply, including deoxygenated blood in the pulmonary arteries and oxygenated blood from the bronchial arteries. The majority of blood to the lungs is supplied by the low pressure [mean arterial pressure (MAP) 12–16 mmHg] pulmonary arteries and participates in gas exchange. Alternatively, the bronchial artery circuit is higher pressure (MAP 100 mmHg), supplying nutrients to the pulmonary system and does not directly interact with alveolar units (38). The majority of cases with significant hemoptysis arise from the bronchial circulation, with hemoptysis arising from the pulmonary circulation accounting for only 5% of cases (39). The bronchial circulation classically arises from the descending aorta, most commonly at the levels of the T5 and T6 vertebrae with patients having 2–4 bronchial arteries (6). Vascular pedicles supplying the bronchial arteries can also supply the esophagus, mediastinal lymph nodes, and importantly, the spinal cord through a complex anastomotic network (40). Bronchial anatomy is variable and classified based off the number of branches originating from each side of the aorta. Four main configurations have been described after evaluation of 150 cadavers (41). Variant anatomy has been seen with origination elsewhere in the aorta or extra-aortic vessels like the subclavian, thyrocervical, axillary, innominate, superior intercostal, pericardiophrenic, and inferior phrenic arteries (6).
Irrespective of the patient’s anatomy, the mechanism of rupture involves derangements of vessels due to some sort of underlying lung pathology. Acute or chronic inflammation leads to hypertrophy and tortuosity of arteries that increases the risk of bleeding. Prolonged or recurrent inflammation leads to more prominent vascular collateral anastomoses, and subsequent increased blood flow through dilated arteries, increasing the risk of bleeding (42). Additionally, neovascularization occurs from increased production of proangiogenic factors (vascular endothelial growth factor and angiopoietin-1) creating new, thin-walled, fragile vessels that can easily bleed (43-45). Ultimately, the exact pathophysiology causing the vascular derangements is variable based off the underlying etiology.
Approach to hemoptysis
The initial approach to hemoptysis should start with rapid evaluation of ABCs to ensure the patient is protecting their airway, ventilating, and hemodynamically stable. If time allows, a history and physical exam focusing on determining the site or side of bleeding should be performed. Careful consideration of mimics such as hematemesis, epistaxis, and bleeding of the gums or nasopharynx should also occur. Features that should specifically be identified are the amount of bleeding, duration, and associated symptoms. Assessment for concurrent infectious symptoms, recent airway instrumentation, past medical history of pulmonary, cardiac or autoimmune disease, history of tobacco use, and risk factors for tuberculosis can help identify the underlying etiology. Review of medications is imperative to assess for anticoagulant or antiplatelet use that could be helpful in explaining the cause.
Laboratory evaluation with a type and cross, complete blood count, coagulation studies, liver and kidney function and arterial blood gas should be obtained and can provide critical information on potential etiologies to guide treatment.
Imaging with a chest radiograph is usually the most readily available imaging modality and can provide valuable information—especially when able to compare with any previous imaging. It is rare for life threatening hemoptysis to occur in the setting of a normal chest radiograph with aspirated blood manifesting as pulmonary opacities (46). While a chest radiograph can provide valuable information to help identify location of bleeding, computed tomography angiography (CTA) of the chest is a more sensitive tool to determine both site and cause of bleeding with success rates of 70% and 77% respectively (3). Other studies have reported that chest radiograph identified the bleeding site in 46% of life-threatening hemoptysis cases and cause in 35%, while chest tomography (CT) correctly localized the site of bleeding in 70–88.5% of cases (47,48). Review of previous imaging, if available, can be useful to help guide a diagnostic plan and potentially negate the need for real time imaging if recent imaging has been obtained. Regardless of which imaging modality is pursued, ensuring patient stability prior to transfer to radiology is imperative. Securement of airway or placement of an endobronchial blocker may be required prior to pursuit of imaging.
Localization of bleeding can be more difficult with chest imaging in patients with pre-existing lung disease and parenchymal abnormalities. Additionally, in patients that are unstable, it may not be feasible to travel to the CT scanner. In these situations, bronchoscopy may be more beneficial to identify the site of bleeding. There have been a variety of studies comparing the efficacy of CT to bronchoscopy. A 2002 study showed that CT localized the site of bleeding in 70% of patients compared to 73% by bronchoscopy, with CT determining the etiology in 77% of cases compared to 8% with bronchoscopy (47). Another prospective study of 606 patients with any amount of hemoptysis revealed that CT diagnosed the underlying cause in 77.3% compared to 48.7% diagnosed with bronchoscopy (25). However, this study included patients with any amount of hemoptysis and likely underestimates the true diagnostic abilities for bronchoscopy in patients with more severe or life-threatening hemoptysis. CT also has the ability to reveal vasculature and surrounding structures to airways which could ultimately help to localize and determine the etiology. In a small study of 40 patients with hemoptysis that had a normal bronchoscopy, subsequent CT of the chest detected an etiology in 50% (49). A recent meta-analysis of studies comparing chest CT to bronchoscopy in 4,635 patients revealed that bronchoscopy had a lower overall diagnostic accuracy in identifying the etiology compared with CT (50). CTA can also improve success of bronchial artery embolization (BAE). A comparative retrospective study of 400 patients with hemoptysis found that those who underwent CTA prior to BAE were more likely to have successful resolution of hemorrhage from embolization and were less likely to need emergent surgery (51). Ultimately, patient characteristics, resources and expertise availability will guide diagnostic choice, but favor CT in clinically stable patients with its ability to better identify the site and underlying etiology. Alternatively, in patients with life threatening emergencies, we favor bronchoscopy due to its ability to secure the airway and stabilize the patient.
Management
There are a variety of strategies available in the management of a patient presenting with hemoptysis. Decision on which method to employ should be quickly considered, while performing a history if time allows, obtaining IV access, and assurance of airway and hemodynamic stability. Determining the side of bleeding is extremely important, as patients can be placed in the lateral decubitus position with the bleeding side dependent, to reduce the risk of aspiration and asphyxiation from blood spilling into the contralateral lung.
Airway stabilization
For patients presenting with life threatening hemoptysis, endotracheal intubation should be considered in patients unable to maintain adequate oxygenation or ventilation, with altered mentation, or inability to expectorate. Ultimately, endotracheal intubation should only occur to establish an airway if a patient is unable to maintain themselves. Endotracheal tube (ETT) placement with an inner diameter of ≥8.5 mm is recommended, to allow for easier insertion of a therapeutic bronchoscope. In patients with significant amounts of blood in the posterior oropharynx that limit visualization during intubation, large-bore suction catheters such as the DuCanto catheter have been shown to provide superior suction capabilities, compared to Yankauer and standard suction catheters (52). Once the airway is secured, isolation of the bleeding lung should occur. Mainstem intubation of the right or left bronchus, contralateral to the bleeding, can be performed to isolate the good lung from hemorrhage. While the left mainstem bronchus is amenable to this approach due to its longer anatomy, the right mainstem is shorter where the right upper lobe bronchus can be easily occluded with the ETT balloon—significantly limiting oxygenation and ventilation to the right middle and lower lobes. One downside to mainstem occlusion is the inability to access the affected lung for therapeutic intervention with a bronchoscope (53,54).
Dual lumen endotracheal tubes (DLET) are designed to isolate each lung and are commonly utilized in thoracic surgery. DLET placement is difficult, with a failure rate recently cited as high as 36% in an OR setting by anesthesiologists, let alone in critically ill patients (2,55). Additionally, their small lumens preclude the ability to utilize standard sized bronchoscopes with large working channels, or allow efficient evacuation of clots from the airways in patients with life-threatening hemoptysis. Due to these technical barriers, intubation with a single lumen ETT is recommended with only 7% of physicians advocating for the insertion of a DLET, in a survey of physician perspectives on hemoptysis management (55). With the limited options of a DLET, we do not recommend placement in patients presenting with life threatening hemoptysis.
Inflatable bronchial blockers or Fogarty balloons are extremely useful in life-threatening hemoptysis, allowing balloon occlusion of a bleeding segment (Figure 1). Subsequent balloon inflation in the bleeding segment has two primary advantages. First, it allows control of bleeding to a fixed volume by providing back pressure to assist with tamponade and hemostasis. It also will contain bleeding to one area to allow ventilation to the rest of the lung (6,15). When utilizing endobronchial blockers, it is important to consider the size of the ETT and diameter of the bronchoscope to not impair ventilation and oxygenation. The combined diameter of the endobronchial blocker and bronchoscope should ideally be less than 1–2 mm smaller than the inner diameter of the ETT (2). If inadequate ventilation or oxygenation occurs, the balloon of the endobronchial blocker should be deflated and withdrawn along with the bronchoscope.
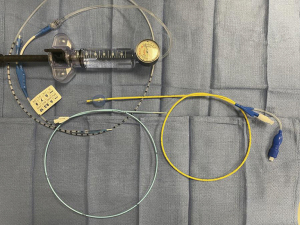
There are a variety of inflatable bronchial blockers available for patients presenting with hemoptysis. Fogarty and CRE balloons are both inserted through the bronchoscope. While these options are easier to adequately place, they limit the scopes ability to suction and navigate the airway with the balloon deployed. CRE balloons are longer and can easily occlude other segments of the lung, while Fogarty balloons are shorter and provide more localized control. The Arndt blocker is lassoed to the outside of the scope and allows more scope mobility. Placement of the balloon can be more difficult due to its inability to be placed through the working channel, but can be left in place for temporization while definitive therapy is pursued.
Bronchoscopy
Flexible bronchoscopy is a valuable tool in patients presenting with hemoptysis that can be conveniently performed at the bedside in the intensive care unit, with a variety of functions (Table 2). This technique is extremely versatile and can be used to lateralize/localize the bleed, identify the etiology, and be used to perform multiple therapeutic interventions. As mentioned previously, bronchoscopy has a lower diagnostic accuracy in determining the etiology of hemoptysis compared to CT, but similar effectiveness in lateralizing the bleed (47,50). While the timing of bronchoscopy is controversial, 84% of pulmonologists prefer bronchoscopy immediately or within the first 24 hours (56), however significant advancement has occurred in interventional radiology since these studies occurred (57,58). In patients that are too unstable to undergo CT scan and require emergent intubation, flexible bronchoscopy is the modality of choice to lateralize the bleed and allow subsequent therapeutic intervention.
Table 2
| Topical |
| Iced cold saline irrigation |
| Endobronchial epinephrine/norepinephrine |
| Vasopressin/desmopressin |
| Thrombin or fibrinogen-thrombin complex |
| Tranexamic acid |
| Endobronchial |
| Balloon tamponade |
| Laser |
| Argon plasma coagulation |
| Cryotherapy |
| Endobronchial valve |
In patients with life-threatening hemoptysis flexible bronchoscopy should be performed rapidly, with the primary goal of localizing the bleed. While views can be limited, suction and advancement of the scope is imperative. If bleeding is found to be in more distal airways, the flexible bronchoscope can be wedged into the bleeding segment to provide tamponade to the local area and allow blood clot formation. While flexible bronchoscopy is an extremely valuable tool, the visual field can become easily obscured with significant bleeding and has limited suction capabilities.
Rigid bronchoscopy is another option for management and, in experienced hands, offers several advantages to the flexible bronchoscope. The large bore allows superior suction capabilities to flexible bronchoscopy allowing for rapid suctioning of blood and removal of clots (Figure 2). The rigid scope also allows simultaneous use of the flexible scope, balloon catheters, and selective lung ventilation. While rigid bronchoscopy affords some significant advantages, it requires more technical skill, cannot be performed at the bedside, and only 17% of pulmonologists prefer it as the initial evaluation with bronchoscopy in one study (56). Alternatively, some institutions prefer urgent bronchoscopy in the operating room, with both flexible and rigid bronchoscopes available, for patients presenting with life-threatening hemoptysis (6). Another approach utilized by both thoracic surgeons and interventional pulmonologists is a combined technique with suspension laryngoscopy which can allow for simultaneous flexible bronchoscopy with the use of rigid bronchoscopy instruments.

A variety of adjuncts to bronchoscopy are available to assist with hemorrhage control. Cold saline is one of the most commonly used, and causes local vasoconstriction. Lavage of ice-cold saline at large volumes allows removal of clots, improves oxygenation, and slows bleeding (6). Conlan and Hurwitz utilized cold saline in the management of 12 patients with life threatening hemoptysis by instilling 50 mL aliquots of normal saline at 4 degrees Celsius through a rigid bronchoscope into the affected lung. The average volume of cold saline used was 500 mL, with all patients experiencing hemostasis, and only one experiencing a complication of bradycardia (59). A similar approach has been described by instilling large aliquots (10–20 mL) of iced saline, leaving it in for 10–15 s, then rapidly removing with suction (6). This process is performed alternating between ventilation, lavage, and then back to ventilation and ultimately allows a large amount of fluid to be irrigated through the lungs (6). Another study of 94 patients with massive hemoptysis underwent ice-cold saline lavage with hemostasis initially achieved in 100% of cases. Twenty of the 79 patients that were followed up experienced recurrent hemoptysis (60). While there have been no randomized controlled trials performed to assess the true effectiveness of iced cold saline, its significant anecdotal success makes it commonly used at most centers for patients with hemoptysis.
Instillation of vasoactive agents such as epinephrine, vasopressin, and desmopressin into the airway are alternative adjuncts to help provide hemostasis by way of local vasoconstriction. Epinephrine is typically diluted in normal saline, and has been shown to be effective in conjunction with cold saline in a series of patients with endobronchial bleeding (61). The dose and dilution of epinephrine that can be administered safely is variably reported in the literature, and requires close monitoring for arrhythmias. Ranges of 0.5 to 20 mL of 1:1,000 to 1:100,000 have been reported with a maximum dose of 0.6 mg suggested (2,62). Unfortunately, there are no randomized controlled trials comparing epinephrine to placebo to understand its true effectiveness in controlling hemorrhage and the effect at any given dose or dilution can be unpredictable. Reports of arrythmia and coronary vasospasm have been described as potential complications in patients receiving intrabronchial epinephrine (62,63). In patients with underlying coronary disease or at high risk for arrythmia, use of norepinephrine to minimize the chronotropic effect has been proposed (62). Additionally, some believe that profuse hemorrhage can wash away epinephrine from the area of interest prior to its ability to provide local vasoconstriction (5,15). Vasopressin administered either intravenous or endobronchial has been shown to be effective in patients with hemoptysis (64,65), as well as desmopressin in a series of patients presenting with hemoptysis related leptospirosis (66).
Thrombin or fibrinogen-thrombin solutions can also be administered through a flexible bronchoscope to provide short-term hemostasis in patients presenting with severe hemoptysis. Tsukamoto and colleagues reported a series of 33 patients with hemoptysis, where 19 cases received thrombin and 14 fibrinogen-thrombin complex infusion. Thrombin was found to be somewhat effective or very effective in 15 of the 19 cases, and fibrinogen-thrombin found to be somewhat effective or very effective in all 14 cases (67). Very effective was defined as no recurrence within 14 days and somewhat effective defined as if hemoptysis recurred after 24 hours, but less than 14 days. More recently, de Gracia et al. performed a prospective observational study of patients presenting with hemoptysis of >150 mL/12 hours and found that in 11 patients where BAE was ineffective, instillation of fibrinogen-thrombin controlled bleeding immediately in 100% of patients (68).
Tranexamic acid (TXA) is an antifibrinolytic that competitively inhibits plasminogen from binding to activators and ultimately inhibits fibrinolysis. While the majority of data for its use is in non-massive hemoptysis, endobronchial, nebulized, and IV administration of TXA has been shown to be effective in the treatment of hemoptysis (69-72). A recent double-blind, randomized controlled trial comparing endobronchial TXA to epinephrine in patients presenting with hemoptysis, not controlled with cold saline, revealed no difference in the average time to control of bleeding (73). Patients either received 1 mg of epinephrine or 500 mg of TXA that were diluted in 20 mL of normal saline and instilled through the bronchoscope 3 times at 90 second intervals, if necessary. Another recent pilot study compared the difference of nebulized TXA to IV TXA in patients presenting to the emergency department (ED) with hemoptysis and found that the incidence of hemoptysis cessation at 30 minutes was significantly higher in the nebulized arm compared to the IV arm (74). Patients received 500 mg three times a day of nebulized or IV TXA with patients in the nebulized TXA arm having a lower need for BAE and higher discharge rate from the ED.
Endobronchial administration of TXA has also been assessed prophylactically in patients undergoing transbronchial lung biopsies (75). In a randomized, double-blind, placebo-controlled trial, patients undergoing transbronchial lung biopsies were randomized to receive 500 mg of TXA diluted in 10 mL of saline or placebo in the lobar bronchus prior to biopsies. Bleeding was found to be significantly less in the patients receiving prophylactic TXA, with no significant adverse events in either group. A recent systematic review and meta-analysis of patients receiving TXA for hemoptysis, found that TXA administration was associated with a reduction in short-term mortality, shorter bleeding time, shorter length of hospital stay, and lower need for intervention with no increase in major or minor adverse events when compared with controls (76). There are a variety of other modalities for local hemostasis in hemoptysis that include absorbable gelatin and thrombin slurries, oxidized regenerated cellulose mesh, recombinant activated factor VII, silicone spigots, polymer surgical sealants, and stents that have been reported with variable success (77-82). We favor a combination of ice-cold saline and endobronchial TXA as adjuncts for hemorrhage control, due to its effectiveness and favorable safety profile.
In situations where hemoptysis originates from more proximal airways, there are a variety of adjuncts available to assist with hemorrhage control. As previously described, inflatable bronchial blockers or Fogarty balloons are extremely useful in life threatening hemoptysis, allowing balloon occlusion of a bleeding segment. Argon plasma coagulation (APC) is another modality utilized and can be used in conjunction with flexible or rigid bronchoscopy. A retrospective review of 31 patients who underwent APC therapy for hemoptysis revealed 100% of patients had immediate resolution of bleeding with no recurrence from treated sites during a follow up duration of over 3 months (83). Six of these patients had bleeding quantified as >200 mL in 24 hours.
In patients with significant airway obstruction from clot, a flexible cryoprobe can be used. A recent series of 16 patients suffering from severe airway obstruction from blood clots revealed successful extraction with a flexible cryoprobe over 27 cases, with only 2 cases requiring rigid bronchoscopy (84). In several cases, endobronchial valves have also been described in patients with refractory hemoptysis where hemostasis was achieved with the valve left in place for as little as 2 weeks (85-87). The Nd:YAG laser is a useful tool for patients with an endobronchial tumor causing hemoptysis allowing the ability to cauterize the site of bleeding, debride tumor, and open partially obstructed airways. In a retrospective review of 52 patients with malignant tracheobronchial lesions and hemoptysis, 77% of patients had resolution of their hemoptysis with reduction in bleeding in an additional 17% (88). While the majority of these modalities have been effective in treating hemoptysis, they are limited to the more proximal, larger airways.
BAE
Once airway stabilization and initial hemostasis has occurred, definitive therapy is required. BAE is a mainstay of therapy that is highly effective, having been first introduced in 1973 for the treatment of massive hemoptysis (89). Ideally, BAE should occur after initial patient stabilization, bronchoscopic intervention to control bleeding, and CT of the chest. With advancements in technique and catheters, BAE has become the procedure of choice in patients with massive hemoptysis. A recent systematic review by Panda et al., found that BAE was successful in 70–99% of cases with major complications occurring 0–6.6% of the time (90). BAE promotes hemostasis by reducing pressure in the abnormal hypertrophic arterial vessels supplying the area of diseased lung (44).
Localization of the bleed angiographically can be difficult, and time consuming, emphasizing the importance of preprocedural CTA or bronchoscopy to localize bleeding. CTA, if available, allows the interventionalist the opportunity to evaluate for the location and size of bronchial arteries requiring intervention. Access is typically obtained through the femoral artery, with controversy on performing a flush thoracic aortogram to identify origins of bronchial arteries (90,91). Imaging findings on angiography for hemoptysis include enlarged hypertrophied arteries, bronchial artery aneurysm, neovascularity, hyperemia, or active extravasation (92). While it can occur, active extravasation is not frequently seen and should not preclude embolization. Numerous embolic agents are available including gelatin sponge, polyvinyl alcohol, tris-acryl microspheres along with many others (92).
BAE can be a life-saving procedure, but does not come without the potential for complications. The most common complications include temporary back pain, chest pain, dysphagia, and post-embolic syndrome (fever, leukocytosis, and pain), with spinal cord ischemia being the most feared from embolism of the anterior spinal artery (5,92). Two recent systemic reviews cite major complications occurring in 0–6.6% and 0.2% of cases respectively (90,93). Recurrence of hemoptysis can occur after successful embolization due to incomplete embolization, recanalization, collateral vessel formation, or simply progression of the patient’s underlying disease. While previous literature had cited recurrence rates as high as 58% a more recent systematic review cited improvement to 23.7% (93).
Surgery
While surgery was previously the primary treatment modality for patients with life threatening hemoptysis, advancements in bronchoscopy, CT scan, and interventional radiology have pushed operative intervention to being a last resort. Operative intervention carries a high mortality of 10–38%, largely related to patients having severe existing pulmonary disease (94-96). Indications for surgical intervention are not standardized, but typically include failure of conservative measures with bronchoscopy and BAE, iatrogenic pulmonary artery rupture, pulmonary aspergilloma, Tuberculosis, large lung abscesses, and chest wall trauma (94,97-99). Emergency surgery for hemoptysis portends a higher mortality than if performed electively. In a retrospective analysis of 111 patients that underwent surgical lung resection for severe hemoptysis, emergent surgery was associated with a 34% mortality compared to 4% after initial control of hemorrhage; and 0% if performed electively (99). Surgical intervention is typically effective with recurrence rates cited to be between 4.2% and 6.2% with poor outcomes occurring if risk factors include older age, pneumonectomy, alcoholism, presurgical need for blood transfusion, and vasopressors (96,99,100). Common post-operative complications include recurrent hemoptysis, persistent air leak, bronchopleural fistulae, empyema, prolonged mechanical ventilation, and need for tracheostomy (2). With the high risk of mortality associated with surgery and effectiveness and favorable safety profile of more conservative interventions, we favor management with flexible or rigid bronchoscopy for local control, and BAE as first line therapy for definitive management. If surgery is ultimately necessary, the aforementioned techniques can be utilized to temporize the patient and allow for an elective or semi-elective operative intervention after recovery from the initial insult.
Once the patient has stabilized from the initial insult and control of bleeding has occurred, definitive treatment for the underlying etiology is imperative. Anti-fungal treatments for mycetoma, antibiotics for infectious causes—including anti-tuberculosis medications for TB, or radiation and chemotherapy should be considered for oncologic causes.
Tracheo-innomiate fistula bleeding
Hemoptysis related to a tracheo-innominate fistula after tracheostomy is a common cause of hemoptysis in critically ill patients. Bleeding after a recent tracheostomy may represent a herald bleed and requires prompt identification and management. Diagnosis requires a high index of suspicion and can be made with CT angiography or bronchoscopy preferably in an operative room. If massive hemorrhage is visualized, immediate manual compression of the artery towards the sternum and control of airway will stabilize the situation while definitive operative intervention can occur.
While there are multiple existing reviews, our review provides a summary of the most up-to-date evidence available on the workup and management of patients presenting with hemoptysis. We have compiled numerous reviews and articles to provide a more comprehensive discussion of evaluation and management of patients presenting with hemoptysis. Specifically, we have focused on the different bronchoscopic interventions available to operators. While one of the strengths of this review is the updates to the existing literature, the paucity of randomized controlled trials for the different therapeutic options is a limitation to our, and other review articles.
Conclusions
Life threatening hemoptysis is a medical emergency with a high morbidity and mortality and requires prompt identification. There are a variety of etiologies of hemoptysis, chest imaging with CT or CTA, or flexible bronchoscopy can help identify the underlying cause. A multidisciplinary team of intensivists, interventional pulmonologists, interventional radiologists, and occasionally surgeons is imperative for appropriate diagnosis and management. Airway stabilization is the first step, with subsequent efforts to identify the side of bleeding with chest CTA or bronchoscopy and its adjuncts to achieve local hemorrhage control depending on the clinical scenario. Definitive management with BAE is the first-line treatment, with surgery being reserved for refractory cases.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathan Kurman and Bryan S. Benn) for the series “Diagnostic & Therapeutic Bronchoscopy” published in AME Medical Journal. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-99/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-99/coif). The series “Diagnostic & Therapeutic Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. L.P. is a consultant for Olympus of America, and has received honoraria and speakers’ fees from that entity. These payments were made directly to him. M.R.P.J. is a consultant for Veran medical, Olympus and Intuitive and has received honoraria and speakers’ fees from that entity. These payments were made directly to him. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thompson AB, Teschler H, Rennard SI. Pathogenesis, evaluation, and therapy for massive hemoptysis. Clin Chest Med 1992;13:69-82. [Crossref] [PubMed]
- Charya AV, Holden VK, Pickering EM. Management of life-threatening hemoptysis in the ICU. J Thorac Dis 2021;13:5139-58. [Crossref] [PubMed]
- Crocco JA, Rooney JJ, Fankushen DS, et al. Massive hemoptysis. Arch Intern Med 1968;121:495-8. [Crossref] [PubMed]
- Garzon AA, Gourin A. Surgical management of massive hemoptysis. A ten-year experience. Ann Surg 1978;187:267-71. [Crossref] [PubMed]
- Davidson K, Shojaee S. Managing Massive Hemoptysis. Chest 2020;157:77-88. [Crossref] [PubMed]
- Reid MM, Reisenauer CJ, Wigle DA, et al. Investigation and Management of Massive Hemoptysis. In Darling G, Baumgartner W, Jacobs J. editors. Pearson's General Thoracic Surgery. STS Cardiothoracic Surgery E-Book. Chicago: Society of Thoracic Surgeons, 2022.
- Hemoptysis Corder R. Emerg Med Clin North Am 2003;21:421-35. [Crossref] [PubMed]
- Fartoukh M, Khoshnood B, Parrot A, et al. Early prediction of in-hospital mortality of patients with hemoptysis: an approach to defining severe hemoptysis. Respiration 2012;83:106-14. [Crossref] [PubMed]
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008;32:1131-2. [Crossref] [PubMed]
- Susanto I. Managing a patient with hemoptysis. Journal of Bronchology 2002;9:40-5. [Crossref]
- Hirshberg B, Biran I, Glazer M, et al. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest 1997;112:440-4. [Crossref] [PubMed]
- Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci 1987;294:301-9. [Crossref] [PubMed]
- Arooj P, Bredin E, Henry MT, et al. Bronchoscopy in the investigation of outpatients with hemoptysis at a lung cancer clinic. Respir Med 2018;139:1-5. [Crossref] [PubMed]
- Kvale PA, Simoff M, Prakash UB, et al. Lung cancer. Palliative care. Chest 2003;123:284S-311S. [Crossref] [PubMed]
- Prey B, Francis A, Williams J, et al. Evaluation and Treatment of Massive Hemoptysis. Surg Clin North Am 2022;102:465-81. [Crossref] [PubMed]
- Miller RR, McGregor DH. Hemorrhage from carcinoma of the lung. Cancer 1980;46:200-5. [Crossref] [PubMed]
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010;80:38-58. [Crossref] [PubMed]
- Lee BR, Yu JY, Ban HJ, et al. Analysis of patients with hemoptysis in a tertiary referral hospital. Tuberc Respir Dis (Seoul) 2012;73:107-14. [Crossref] [PubMed]
- Ong TH, Eng P. Massive hemoptysis requiring intensive care. Intensive Care Med 2003;29:317-20. [Crossref] [PubMed]
- Reechaipichitkul W, Latong S. Etiology and treatment outcomes of massive hemoptysis. Southeast Asian J Trop Med Public Health 2005;36:474-80. [PubMed]
- Chan VL, So LK, Lam JY, et al. Major haemoptysis in Hong Kong: aetiologies, angiographic findings and outcomes of bronchial artery embolisation. Int J Tuberc Lung Dis 2009;13:1167-73. [PubMed]
- Bhalla A, Pannu AK, Suri V. Etiology and outcome of moderate-to-massive hemoptysis: Experience from a tertiary care center of North India. Int J Mycobacteriol 2017;6:307-10. [Crossref] [PubMed]
- Abal AT, Nair PC, Cherian J. Haemoptysis: aetiology, evaluation and outcome--a prospective study in a third-world country. Respir Med 2001;95:548-52. [Crossref] [PubMed]
- Dweik RA, Stoller JK. Role of bronchoscopy in massive hemoptysis. Clin Chest Med 1999;20:89-105. [Crossref] [PubMed]
- Mondoni M, Carlucci P, Job S, et al. Observational, multicentre study on the epidemiology of haemoptysis. Eur Respir J 2018;51:1701813. [Crossref] [PubMed]
- Cahill BC, Ingbar DH. Massive hemoptysis. Assessment and management. Clin Chest Med 1994;15:147-67. [Crossref] [PubMed]
- Razazi K, Parrot A, Khalil A, et al. Severe haemoptysis in patients with nonsmall cell lung carcinoma. Eur Respir J 2015;45:756-64. [Crossref] [PubMed]
- Bernasconi M, Koegelenberg CFN, Koutsokera A, et al. Iatrogenic bleeding during flexible bronchoscopy: risk factors, prophylactic measures and management. ERJ Open Res 2017;3:00084-2016. [Crossref] [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [Crossref] [PubMed]
- Facciolongo N, Patelli M, Gasparini S, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis 2009;71:8-14. [PubMed]
- Herth FJ, Becker HD, Ernst A. Aspirin does not increase bleeding complications after transbronchial biopsy. Chest 2002;122:1461-4. [Crossref] [PubMed]
- Ernst A, Eberhardt R, Wahidi M, et al. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest 2006;129:734-7. [Crossref] [PubMed]
- Papin TA, Lynch JP 3rd, Weg JG. Transbronchial biopsy in the thrombocytopenic patient. Chest 1985;88:549-52. [Crossref] [PubMed]
- Wahidi MM, Rocha AT, Hollingsworth JW, et al. Contraindications and safety of transbronchial lung biopsy via flexible bronchoscopy. A survey of pulmonologists and review of the literature. Respiration 2005;72:285-95. [Crossref] [PubMed]
- Savale L, Parrot A, Khalil A, et al. Cryptogenic hemoptysis: from a benign to a life-threatening pathologic vascular condition. Am J Respir Crit Care Med 2007;175:1181-5. [Crossref] [PubMed]
- Ando T, Kawashima M, Masuda K, et al. Clinical and Angiographic Characteristics of 35 Patients With Cryptogenic Hemoptysis. Chest 2017;152:1008-14. [Crossref] [PubMed]
- Kervancioglu S, Bayram N, Gelebek Yilmaz F, et al. Radiological findings and outcomes of bronchial artery embolization in cryptogenic hemoptysis. J Korean Med Sci 2015;30:591-7. [Crossref] [PubMed]
- Walker CM, Rosado-de-Christenson ML, Martínez-Jiménez S, et al. Bronchial arteries: anatomy, function, hypertrophy, and anomalies. Radiographics 2015;35:32-49. [Crossref] [PubMed]
- Lordan JL, Gascoigne A, Corris PA. The pulmonary physician in critical care * Illustrative case 7: Assessment and management of massive haemoptysis. Thorax 2003;58:814-9. [Crossref] [PubMed]
- Fraser KL, Grosman H, Hyland RH, et al. Transverse myelitis: a reversible complication of bronchial artery embolisation in cystic fibrosis. Thorax 1997;52:99-101. [Crossref] [PubMed]
- Cauldwell EW, Siekert RG. The bronchial arteries; an anatomic study of 150 human cadavers. Surg Gynecol Obstet 1948;86:395-412. [PubMed]
- Deffebach ME, Charan NB, Lakshminarayan S, et al. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987;135:463-81. [PubMed]
- Larici AR, Franchi P, Occhipinti M, et al. Diagnosis and management of hemoptysis. Diagn Interv Radiol 2014;20:299-309. [Crossref] [PubMed]
- Kathuria H, Hollingsworth HM, Vilvendhan R, et al. Management of life-threatening hemoptysis. J Intensive Care 2020;8:23. [Crossref] [PubMed]
- McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med 2001;164:S39-45. [Crossref] [PubMed]
- McCollun WB, Mattox KL, Guinn GA, et al. Immediate operative treatment for massive hemoptysis. Chest 1975;67:152-5. [Crossref] [PubMed]
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002;179:1217-24. [Crossref] [PubMed]
- Khalil A, Fedida B, Parrot A, et al. Severe hemoptysis: From diagnosis to embolization. Diagn Interv Imaging 2015;96:775-88. [Crossref] [PubMed]
- Millar AB, Boothroyd AE, Edwards D, et al. The role of computed tomography (CT) in the investigation of unexplained haemoptysis. Respir Med 1992;86:39-44. [Crossref] [PubMed]
- He D, Huang D, Huang K, et al. The Efficacy of Bronchoscopy versus Computerised Tomography in Initial Identification of Patients with Hemoptysis. J Coll Physicians Surg Pak 2021;31:1459-67. [Crossref] [PubMed]
- Khalil A, Fartoukh M, Parrot A, et al. Impact of MDCT angiography on the management of patients with hemoptysis. AJR Am J Roentgenol 2010;195:772-8. [Crossref] [PubMed]
- Finke SR, Schroeder DC, Ecker H, et al. Comparing suction rates of novel DuCanto catheter against Yankauer and standard suction catheter using liquids of different viscosity-a technical simulation. BMC Anesthesiol 2022;22:285. [Crossref] [PubMed]
- Gagnon S, Quigley N, Dutau H, et al. Approach to Hemoptysis in the Modern Era. Can Respir J 2017;2017:1565030. [Crossref] [PubMed]
- Håkanson E, Konstantinov IE, Fransson SG, et al. Management of life-threatening haemoptysis. Br J Anaesth 2002;88:291-5. [Crossref] [PubMed]
- Campos JH, Hallam EA, Van Natta T, et al. Devices for lung isolation used by anesthesiologists with limited thoracic experience: comparison of double-lumen endotracheal tube, Univent torque control blocker, and Arndt wire-guided endobronchial blocker. Anesthesiology 2006;104:261-6, discussion 5A. [Crossref] [PubMed]
- Haponik EF, Chin R. Hemoptysis: clinicians' perspectives. Chest 1990;97:469-75. [Crossref] [PubMed]
- Torbiarczyk JM, Sobczak PA, Torbiarczyk KK, et al. Is bronchoscopy always justified in diagnosis of haemoptysis? Adv Respir Med 2018;86:13-6. [Crossref] [PubMed]
- Hsiao EI, Kirsch CM, Kagawa FT, et al. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001;177:861-7. [Crossref] [PubMed]
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980;35:901-4. [Crossref] [PubMed]
- Marsico GA, Guimarães CA, Montessi J. Costa AMMd, Madeira L. Management of massive hemoptysis with the rigid bronchoscope and cold saline lavage. Jornal de Pneumologia 2003;29:280-6. [Crossref]
- Düpree HJ, Lewejohann JC, Gleiss J, et al. Fiberoptic bronchoscopy of intubated patients with life-threatening hemoptysis. World J Surg 2001;25:104-7. [Crossref] [PubMed]
- Khoo KL, Lee P, Mehta AC. Endobronchial epinephrine: confusion is in the air. Am J Respir Crit Care Med 2013;187:1137-8. [Crossref] [PubMed]
- Steinfort DP, Herth FJ, Eberhardt R, et al. Potentially fatal arrhythmia complicating endobronchial epinephrine for control of iatrogenic bleeding. Am J Respir Crit Care Med 2012;185:1028-30. [Crossref] [PubMed]
- Breuer HW, Charchut S, Worth H, et al. Endobronchial versus intravenous application of the vasopressin derivative glypressin during diagnostic bronchoscopy. Eur Respir J 1989;2:225-8. [Crossref] [PubMed]
- Bilton D, Webb AK, Foster H, et al. Life threatening haemoptysis in cystic fibrosis: an alternative therapeutic approach. Thorax 1990;45:975-6. [Crossref] [PubMed]
- Pea L, Roda L, Boussaud V, et al. Desmopressin therapy for massive hemoptysis associated with severe leptospirosis. Am J Respir Crit Care Med 2003;167:726-8. [Crossref] [PubMed]
- Tsukamoto T, Sasaki H, Nakamura H. Treatment of hemoptysis patients by thrombin and fibrinogen-thrombin infusion therapy using a fiberoptic bronchoscope. Chest 1989;96:473-6. [Crossref] [PubMed]
- de Gracia J, de la Rosa D, Catalán E, et al. Use of endoscopic fibrinogen-thrombin in the treatment of severe hemoptysis. Respir Med 2003;97:790-5. [Crossref] [PubMed]
- Márquez-Martín E, Vergara DG, Martín-Juan J, et al. Endobronchial administration of tranexamic Acid for controlling pulmonary bleeding: a pilot study. J Bronchology Interv Pulmonol 2010;17:122-5. [Crossref] [PubMed]
- Wand O, Guber E, Guber A, et al. Inhaled Tranexamic Acid for Hemoptysis Treatment: A Randomized Controlled Trial. Chest 2018;154:1379-84. [Crossref] [PubMed]
- Bellam BL, Dhibar DP, Suri V, et al. Efficacy of tranexamic acid in haemoptysis: A randomized, controlled pilot study. Pulm Pharmacol Ther 2016;40:80-3. [Crossref] [PubMed]
- Zamani A. Bronchoscopic intratumoral injection of tranexamic acid to prevent excessive bleeding during multiple forceps biopsies of lesions with a high risk of bleeding: a prospective case series. BMC Cancer 2014;14:143. [Crossref] [PubMed]
- Fekri MS, Hashemi-Bajgani SM, Shafahi A, et al. Comparing Adrenaline with Tranexamic Acid to Control Acute Endobronchial Bleeding: A Randomized Controlled Trial. Iran J Med Sci 2017;42:129-35. [PubMed]
- Gopinath B, Mishra PR, Aggarwal P, et al. Nebulized vs IV Tranexamic Acid for Hemoptysis: A Pilot Randomized Controlled Trial. Chest 2023;163:1176-84. [Crossref] [PubMed]
- Kuint R, Levy L, Cohen Goichman P, et al. Prophylactic use of tranexamic acid for prevention of bleeding during transbronchial lung biopsies - A randomized, double-blind, placebo-controlled trial. Respir Med 2020;173:106162. [Crossref] [PubMed]
- Chen LF, Wang TC, Lin TY, et al. Does tranexamic acid reduce risk of mortality on patients with hemoptysis?: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e25898. [Crossref] [PubMed]
- Valipour A, Kreuzer A, Koller H, et al. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005;127:2113-8. [Crossref] [PubMed]
- Peralta AR, Chawla M, Lee RP. Novel Bronchoscopic Management of Airway Bleeding With Absorbable Gelatin and Thrombin Slurry. J Bronchology Interv Pulmonol 2018;25:204-11. [Crossref] [PubMed]
- Bhattacharyya P, Dutta A, Samanta AN, et al. New procedure: bronchoscopic endobronchial sealing; a new mode of managing hemoptysis. Chest 2002;121:2066-9. [Crossref] [PubMed]
- Bylicki O, Vandemoortele T, Laroumagne S, et al. Temporary endobronchial embolization with silicone spigots for moderate hemoptysis: a retrospective study. Respiration 2012;84:225-30. [Crossref] [PubMed]
- Lee SA, Kim DH, Jeon GS. Covered bronchial stent insertion to manage airway obstruction with hemoptysis caused by lung cancer. Korean J Radiol 2012;13:515-20. [Crossref] [PubMed]
- Heslet L, Nielsen JD, Levi M, et al. Successful pulmonary administration of activated recombinant factor VII in diffuse alveolar hemorrhage. Crit Care 2006;10:R177. [Crossref] [PubMed]
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Schmidt LH, Schulze AB, Goerlich D, et al. Blood clot removal by cryoextraction in critically ill patients with pulmonary hemorrhage. J Thorac Dis 2019;11:4319-27. [Crossref] [PubMed]
- Frey JW, Postigo M, Pitts LR. Endobronchial Valve Placement as Salvage Therapy in the Management of Hemoptysis. J Bronchology Interv Pulmonol 2023;30:301-3. [Crossref] [PubMed]
- Patel B, Abi-Fadel D, Rosenheck J, et al. Endobronchial Valves for Treatment of Hemoptysis. J Bronchology Interv Pulmonol 2019;26:e22-4. [Crossref] [PubMed]
- Lalla U, Allwood BW, Sinha Roy S, et al. Endobronchial Valve Used as Salvage Therapy in a Mechanically Ventilated Patient with Intractable Life-Threatening Haemoptysis. Respiration 2017;93:436-40. [Crossref] [PubMed]
- Han CC, Prasetyo D, Wright GM. Endobronchial palliation using Nd:YAG laser is associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol 2007;2:59-64. [Crossref] [PubMed]
- Remy J, Voisin C, Ribet M, et al. Treatment, by embolization, of severe or repeated hemoptysis associated with systemic hypervascularization. Nouv Presse Med 1973;2:2060. [PubMed]
- Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol 2017;23:307-17. [Crossref] [PubMed]
- Yoon W, Kim JK, Kim YH, et al. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395-409. [Crossref] [PubMed]
- Kaufman CS, Kwan SW. Bronchial Artery Embolization. Semin Intervent Radiol 2022;39:210-7. [Crossref] [PubMed]
- Zheng Z, Zhuang Z, Yang M, et al. Bronchial artery embolization for hemoptysis: A systematic review and meta-analysis. J Interv Med 2021;4:172-80. [Crossref] [PubMed]
- Endo S, Otani S, Saito N, et al. Management of massive hemoptysis in a thoracic surgical unit. Eur J Cardiothorac Surg 2003;23:467-72. [Crossref] [PubMed]
- Ayed A. Pulmonary resection for massive hemoptysis of benign etiology. Eur J Cardiothorac Surg 2003;24:689-93. [Crossref] [PubMed]
- Kiral H, Evman S, Tezel C, et al. Pulmonary resection in the treatment of life-threatening hemoptysis. Ann Thorac Cardiovasc Surg 2015;21:125-31. [Crossref] [PubMed]
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000;28:1642-7. [Crossref] [PubMed]
- Yun JS, Song SY, Na KJ, et al. Surgery for hemoptysis in patients with benign lung disease. J Thorac Dis 2018;10:3532-8. [Crossref] [PubMed]
- Andréjak C, Parrot A, Bazelly B, et al. Surgical lung resection for severe hemoptysis. Ann Thorac Surg 2009;88:1556-65. [Crossref] [PubMed]
- Brik A, Salem AM, Shoukry A, et al. Surgery for hemoptysis in various pulmonary tuberculous lesions: a prospective study. Interact Cardiovasc Thorac Surg 2011;13:276-9. [Crossref] [PubMed]
Cite this article as: Chandler JK, Reid MM, Pitts L, Postigo Jasahui MR. Evaluation & management of clinically significant hemoptysis—a clinical practice review. AME Med J 2024;9:1.

