Added value of endoscopic ultrasound to endobronchial ultrasound in non-small cell lung cancer staging
Introduction
Lung cancer remains the leading global cause of cancer-related deaths and disability in both men and women (1,2). Because of its high morbidity and mortality, rapid diagnosis and accurate staging is essential in determination of treatment and identification of potential surgical candidates.
Advancements in radiographic imaging and biopsy methods have improved lung cancer staging over the years. Radiographic techniques such as computed tomography (CT) and positron emission tomography-computed tomography (PET-CT) have helped identify suspicious nodules as well as distant metastases. However, tissue sampling is still required for confirmation of malignancy and staging due to high false positive results with imaging alone (3-11).
Historically, the test of choice in sampling mediastinal disease was surgical mediastinoscopy. However, this invasive method requires general anesthesia and has complications including hemorrhage, tracheal injury, pneumothorax, recurrent laryngeal nerve palsy, and a mortality rate of 0.08% (12).
Current guidelines for lung cancer diagnosis and staging recommend endoscopic methods as the first procedure for acquiring tissue (13,14). Since its development, a growing body of literature has shown endobronchial ultrasound (EBUS) to be superior to surgical methods in sampling mediastinal disease including mediastinal masses, lymphadenopathy, and metastatic disease (13,15-18). This minimally invasive technique does not require anesthesia, offers better patient comfort, costs less, and has overall lower risks (19). EBUS has since been utilized as the first procedure in accessing hilar and mediastinal lesions as well as central parenchymal and intrabronchial lesions (19,20). The EBUS scope can be used for transesophageal biopsies (EUS-B), mirroring the endoscopic ultrasound (EUS) technique that is more commonly utilized by gastroenterologists in the United States.
Endoscopic procedures can also expedite time to staging. When combined with the use of rapid on site evaluation (ROSE), providers can obtain rapid confirmation of accurate sampling and receive preliminary pathology results to expedite time to diagnosis (21).
Here, we describe the benefit of combining two minimally invasive ultrasound-guided techniques through endobronchial route (EBUS) and transesophageal route (EUS/EUS-B) in the diagnosis and staging of lung cancers.
Lung cancer staging guidelines
Accurate lung cancer staging is essential in the determination of therapy options and overall prognosis for the patient.
The European Society of Gastrointestinal Endoscopy (ESGE), European Respiratory Society (ERS), European Society of Thoracic Surgeons (ESTS), American College of Chest Physicians (ACCP), and National Comprehensive Cancer Network (NCCN) guidelines outline the different scenarios where tumors and lymph nodes should be sampled (13,14). All central tumors, peripheral tumors >3 cm or lymph nodes >1 cm on CT, N1 lymph node involvement on PET-CT, and any PET positive mediastinal lymph nodes with standardized uptake value (SUV) >2 regardless of node size on PET should be staged. Invasive mediastinal staging is not required in patients with peripheral tumor size <3 cm and without lymph node involvement on CT or PET-CT.
The ACCP currently recommends endoscopic methods as the best first step in evaluating and staging potentially resectable non-small cell lung cancer (NSCLC) (13). These techniques are also recommended for suspected N2 or N3 lymph node involvement. Patients with poor lung function should be staged to identify N1 lymph node metastases prior to planning for stereotactic body radiation therapy (SBRT) or sub-lobar resection (15). Early identification of N2/N3 lymph nodes and distant metastases can prevent futile surgery while identifying those who could benefit from neoadjuvant therapy. The technique used to sample the mediastinum is selected at the discretion of the operator and their skill level (13). In the event that endoscopic biopsies of suspicious nodes are nondiagnostic, the ACCP does recommend surgical sampling.
The endoscopic procedures
The three procedures to be discussed in this paper are EBUS, EUS, and the passage of an ultrasound bronchoscope (EBUS scope) through the esophagus to acquire samples which is called EUS-B. We will collectively refer to the transesophageal approach as EUS/EUS-B. Distinctions will be made when citing references that have studied EUS and/or EUS-B separately.
Background on EBUS and EUS/EUS-B
EBUS is currently the first diagnostic technique used in evaluating centrally located lesions including hilar, mediastinal, central parenchymal, and intrabronchial lesions (19,20). Its diagnostic accuracy and safety profile make EBUS the ideal diagnostic modality (13,14,22).
While not standard practice, an experienced pulmonologist may pass instruments through the esophagus for additional sampling of lesions. EUS-B refers to the technique of passing a linear ultrasound bronchoscope probe through the esophagus to access nearby structures. EUS is a longer probe that is passed through the esophagus to obtain samples most typically within and near the gastrointestinal tract. The EUS and EBUS scopes have differences in their structure that offer advantages. The EUS scope is longer than the EBUS scope and therefore covers more distance and range. However, unlike the EBUS scope, the larger diameter of the EUS scope does not permit its use within the airways. When moderate sedation is used, the smaller EUS-B may be more comfortable and better tolerated than the EUS scope. The linear convex EUS scope has a 180° view that is parallel to the endoscope shaft and can obtain images at a depth of 3 to 8 cm. In comparison, the convex EBUS scope has a 50° to 80° view parallel to the endoscope shaft and obtains images between 2 to 5 cm in depth though this can vary depending on scope type.
Sampling technique
Both EBUS and EUS/EUS-B obtain samples through either fine needle aspirations (FNA) or fine needle biopsies (FNB). Although the 180° view by EUS offers a better imaging quality and range, there has been no statistically significant difference in mediastinal lymph node or left adrenal gland sampling between EUS and EUS-B (23,24). Mangiavillano et al. found that in sampling parenchymal lesions of the lung, FNB was superior in diagnostic accuracy and sensitivity when compared to FNA (25). Both EUS and EUS-B can be performed during the second half of a staging session, allowing more time for the patient to ease out of the sedation from the first half of their procedure. Typically, the sedation requirements are much less with EUS/EUS-B compared to EBUS due to less cough reflex during EUS.
There are a range of FNA and FNB needles produced by medical device companies that are compatible with the working channels of the EBUS or EUS scopes. The EBUS compatible needles have a similar gauge range at 19, 21, 22, and 25 and a working length of 0–5 cm. The more commonly sized needles for use with EUS include 19, 22, and 25 gauge needles with a length ranging from 0–8 cm. The longer needles used with EUS permits sampling of deeper structures.
The techniques for needle aspiration are similar in both EUS/EUS-B and EBUS. The needle is quickly advanced to puncture the lymph node. Once the needle is positioned, quick agitations inside the lymph node acquire sample. During this process, needle movement must be visualized within the lymph node to ensure proper sampling of the target. Poor contact between the ultrasound probe and esophageal wall can be overcome with saline filled balloons with a latex-free option for patients with a latex allergy (26). Despite the improvement in image quality with use of balloons, there are no studies suggesting correlation with improvement in diagnostic yield.
While EUS and EUS-B may appear similar, there are situations where one may be preferred over the other. Due to the increased length of the scope, EUS is better for biopsies of certain sub-diaphragmatic lesions such as the right adrenal gland or right lobe of the liver. In situations where decreased sedation is required or there are higher O2 requirements, the patient may tolerate EUS-B more than EUS, possibly because of the smaller diameter in scope size (27-33).
The order of procedures in combining EBUS and EUS makes a difference on outcomes. Korevaar et al. found that the addition of EBUS to EUS/EUS-B increased sensitivity by 22% while EUS/EUS-B to EBUS increased sensitivity by 12% (34). Hwangbo et al. found that EUS added small additional value to EBUS. When EUS was performed first, more lesions were missed and EBUS had an increased additive value (35). EUS still remained important in upstaging the cancer and diagnosing metastases that were missed by EBUS, specifically with lymph nodes at station 4L and also posteriorly and inferiorly located station 7 (36). Interestingly, the lesions missed by EBUS and picked up by EUS were not located in the inferior mediastinum but in station 4L and 7.
Practical use of EUS/EUS-B
Mediastinal lymph nodes
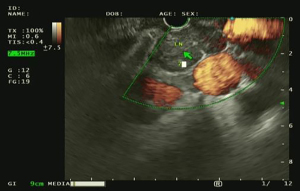
EUS/EUS-B provides access to para-esophageal lymph nodes that have limited or no accessibility by traditional EBUS. Table 1 provides a comparison of sites accessible by EUS/EUS-B and EBUS. Sites better reached by EUS/EUS-B include lesions closer to the esophagus such as lymph nodes in the inferior mediastinum including stations 1, 2R, 2L, 3P, 4L, 5, 6, 7, 8, 9, and 10L (27,39). There are techniques to sample para-aortic station 6 both with and without traversing the aorta (37,38,40). Inferior mediastinal and sub-diaphragmatic nodes and structures that can be sampled by EUS/EUS-B include lymph nodes in station 8 and 9, the celiac axis, left lobe of the liver, bilateral adrenal glands, and the spleen (41). Figure 1 shows a liver mass as seen using EUS.
Table 1
| Lymph nodes/structures | EBUS | EUS-B | EUS |
|---|---|---|---|
| 1: supraclavicular nodes | X | X* | X* |
| 2R: upper paratracheal nodes, right | X | X** | X** |
| 2L: upper paratracheal nodes, left | X | X | X |
| 3A: prevascular nodes | X*** | – | – |
| 3P: retrotracheal nodes | X | X | X |
| 4R: lower paratracheal nodes, right | X | X* | X* |
| 4L: lower paratracheal nodes, left | X | X | X |
| 5: subaortic (AP window) nodes | – | X | X |
| 6: para-aortic nodes | – | X**** | X**** |
| 7: subcarinal nodes | X | X | X |
| 8: paraesophageal nodes | – | X | X |
| 9: pulmonary ligament nodes | – | X | X |
| 10: hilar nodes | X | – | – |
| 11: interlobar nodes | X | – | – |
| Gastrohepatic ligament nodes | – | X | X |
| Celiac axis lymph nodes | – | X | X |
| Left lobe of liver | – | X | X |
| Right lobe of liver | – | – | X |
| Left adrenal gland | – | X | X |
| Right adrenal gland | – | – | X |
“X” indicates that the lymph node station or structure is usually accessible with the specified diagnostic technique. “–” indicates that the lesion is not accessible by the specified technique. *, depending on its location, station 1 may be reached by either EBUS or EUS/EUS-B. **, stations 2R and 4R may be accessible by EUS or EUS-B if the target is large enough. Lymph nodes smaller than 1 cm are usually difficult to access due to the trachea. ***, station 3A is typically inaccessible by EBUS unless using a transvascular approach. ****, station 6 can be accessed with techniques both with and without traversing the aorta. These techniques are described by Liberman et al. (37,38). EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; EUS-B, endoscopic ultrasound through the esophagus using an ultrasound bronchoscope.
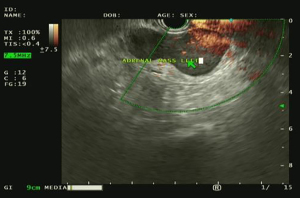
The ease of biopsy can vary depending on the laterality of the lesion. EUS/EUS-B can sample stations 2L and 4L (paratracheal stations), station 7 (subcarinal station), and stations 8 and 9 (inferior mediastinal stations). Figure 2 shows visualization of station 7 by EUS. In their evaluation of patients who underwent combined EBUS and EUS, Wallace et al. found that EUS was superior to EBUS in detecting malignant disease in stations 5, 6, and 7 (42). Assisi et al. found that in patients suspected to have NSCLC, sampling of stations 7–9 had a sensitivity of 90.5% which was higher than that of station 4L (75%) and station 5 (87.5%) (43). Typically, due to interference by the trachea, lymph nodes in station 2R and 4R have limited accessibility. However, if large enough (≥2 cm), lymph nodes in station 4R can also be reached.
Rarely, as many as one in 30 patients with NSCLC may have inferior mediastinal lymph node involvement without involving upper mediastinal lymph nodes (15). Sampling of CT and PET negative lymph nodes and adrenal glands can be upstaged by endoscopy with 8% having skipped adrenal metastases identified by EUS only (44). The expanded range of EUS/EUS-B allows for biopsy of extra-nodal lesions.
Biopsies near major vessels
Frequently, lymph nodes of interest are located near major vessels. Aspirations of airway adjacent lymph nodes sampled through or behind vessels such as the aorta, pulmonary artery, and portal vein are not associated with an increased rate of adverse events (45). Para-aortic station 5 and 6 lymph nodes can also be biopsied by EUS/EUS-B with or without traversing the aorta (37-39). A meta-analysis of 17 studies looked at the safety of EBUS/EUS/EUS-B needle aspiration of thoracic and abdominal lesions behind major vessels including the aorta, pulmonary artery, and portal vein. The authors found a pooled sampling accuracy for EBUS/EUS-B/EUS of 85% with a bleeding risk of 1.4%, all of which were mild and self-resolved (45). A retrospective study evaluated 33 consecutive patients who underwent mediastinal staging through the pulmonary artery or aorta and found an overall yield of 73% with no complications in the immediate post-procedural or 12-month follow-up period (46). In a larger retrospective study of 100 patients, transvascular biopsies were obtained by EUS and EBUS through the aorta and pulmonary artery with a median of 2 passes (47). There were no operative or immediate postoperative complications and only one patient had a delayed complication of aortic pseudoaneurysm which was conservatively managed in the median 12 month follow-up period. The overall sensitivity of the samples from this study was 71.5% with a diagnostic accuracy of 74.5%. Another study by von Bartheld et al. reviewed outcomes in biopsies through the aorta in 14 patients and found a sensitivity of 64–75% with specificity of 100% (48). The lower sensitivity may be due to the lower number of passes and targets biopsied (49,50).
Methods for sampling lymph nodes at station 6 without traversing the aorta have also been described (15,23). In a case series of 12 consecutive patients, station 6 was accessed by passing the needle through the proximal esophagus towards the para-aortic area (51). Successful diagnosis by cytology was made without any immediate or 30-day post-procedural morbidity.
Adrenal glands
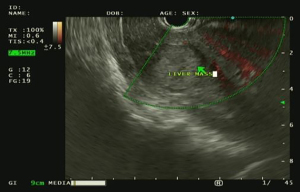
A unique addition of EUS/EUS-B is biopsy of the adrenal glands, a common and sometimes isolated metastatic site of lung cancer (52-54). Both the left and right adrenal glands when visualized by ultrasound are described as having a characteristic “seagull sign” (55). The techniques to sample the left and right adrenal glands are different. To access the left adrenal gland, the EUS/EUS-B scope is passed through the stomach. The back left side is viewed to locate the left kidney and identify the left adrenal gland by its bird-like appearance. Both EUS and EUS-B can be used to biopsy the left adrenal gland. In contrast, the right adrenal gland is accessible only by EUS using a transduodenal approach. The right kidney is identified and the scope withdrawn anterior to and above the right kidney while slowly scanning for the right adrenal gland. In our practice, the left adrenal gland is easier to identify than the right but both are accessible with user experience. Figure 3 shows a mass in the left adrenal gland as identified by EUS.
EUS/EUS-B can be used to sample both the adrenal glands and lung lesions in the same session, providing accurate and expedited staging. Our practice has experience in sampling adrenal glands using EUS. In a case series of 13 patients, the presence or absence of metastatic disease was established in all patients with a diagnostic yield of 100% (56). This is consistent with prior reports (57,58). Our institution also looked at 113 patients who underwent adrenal gland sampling with EUS and showed upstaging in 24% of patients to stage 4 due to presence of metastatic disease in the adrenal glands. Diagnosis by combined procedure can precede abnormal diagnostic imaging as 14% of patients in our study had normal or no PET scan uptake in the adrenal glands but found pathology on endosonography with malignancy on biopsy. In another study, EUS identified skip metastases to the adrenal gland that were not detected on CT and PET imaging (44).
Central and parenchymal tumors
EUS/EUS-B can be used to accurately sample central and parenchymal tumors. In a retrospective study of 55 patients, EUS-FNA had both an accuracy and sensitivity of 94.5% without immediate or 30-day morbidity (59). Another retrospective study based in Italy found that parenchymal lesions difficult to access by EBUS were able to be biopsied by EUS-FNA/FNB with an overall diagnostic accuracy of 88.9% (25). Our facility has had success in obtaining transesophageal lung biopsies of central tumors close to the esophagus. In a sample of 20 patients, adequate tissue sample led to definitive diagnosis in 19 patients with a 95% diagnostic yield with no major procedural complications noted in the immediate 30-day period (56). This is similar to the diagnostic yield reported in previous studies at 95–100% (30,31,59,60).
Patient factors
EUS/EUS-B also provides an option for biopsy in patients who are otherwise poor candidates for a standard bronchoscopy (28). Some patients may not be amenable to bronchoscopy secondary to airway stenosis or respiratory failure (17). EUS-B may have an advantage over EUS in these cases. In a series of 10 patients investigated by Khoury et al., awake EUS-B was performed in patients with higher risk of complications from sedation (29). Scenarios included advanced chronic obstructive pulmonary disease (COPD) with severe hypoxemia, uncontrolled congestive heart failure, severe pulmonary hypertension, severe obstructive sleep apnea, mandibular deformity leading to difficult intubation, and refusal of sedation. Through EUS-B, mediastinal lymph nodes in these patients were safely and successfully sampled without complications. Another study found that EUS-B was tolerated better with a shorter procedure time, fewer oxygen desaturations during the procedure, better operator experience, and required fewer sedatives without compromising on diagnostic accuracy (59).
Efficacy of EUS/EUS-B
EUS/EUS-B has been found to be efficacious in detecting and staging NSCLC. In a study evaluating EUS-FNA of cancers in the mediastinum, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were reported at 92%, 100%, 100% and 80% respectively (61). The pooled sensitivities in two meta-analyses of EUS-FNA in nodal staging of NSCLC were 83% and 89% (13,62).
EUS/EUS-B has demonstrated advantages over surgical approaches for biopsy. In a randomized control trial comparing EUS-FNA with surgical mediastinoscopy in 40 patients, there was no difference in the diagnostic results of the two procedures (63). EUS can also access lesions that are inaccessible by a conventional surgical approach. Without EUS, the AP window and para-aortic area would require surgical sampling not by standard mediastinoscopy but through video-assisted thoracoscopic surgery (VATS), Chamberlain procedure (left anterior mediastinoscopy), or extended cervical mediastinoscopy. Thus, EUS offers the additional advantage of being minimally invasive while acquiring biopsies in areas inaccessible by EBUS and standard mediastinoscopy.
Occult metastases in a radiographically normal mediastinum are detected by EUS-FNA with a sensitivity of 58% (62). In a prospective study evaluating patients with a radiographically normal mediastinum, 2 out of 56 patients were identified with N3 disease using EUS-FNA (64). Similarly, another trial identified 5 out of 47 patients as having N2 disease (65). The additional benefits of EUS/EUS-B in detecting occult malignancy are further detailed in the following section.
Combined EBUS/EUS/EUS-B
Combining EBUS with EUS/EUS-B offers advantages when compared to single procedure alone. Multiple studies have shown additive benefits leading to rapid diagnosis of malignancy and improved staging accuracy.
Combined procedure compared to single procedure
Due to the broadened accessibility of lymph nodes, combined EBUS with EUS/EUS-B can completely stage the mediastinum and include sub-diaphragmatic structures often affected by metastatic disease. Multiple studies have shown that the combination of EBUS with EUS/EUS-B has an increased diagnostic accuracy and sensitivity compared to EBUS alone in patients with proven or suspected lung cancer (14,34,66).
Combined EBUS/EUS/EUS-B has a high sensitivity in detecting mediastinal lymph node metastasis. Liberman et al. described a combined sensitivity of 91% while another study reports a sensitivity of 93%, similar to that reported elsewhere in medical literature (17,18,41,67). One study found combined procedure increased sensitivity by 21% when compared to EUS alone and 13% when compared to EBUS alone (14). A meta-analysis of 13 studies found that the combined approach had a mean sensitivity of 86% and NPV of 92% (34). Another meta-analysis of 10 studies showed that combined EBUS/EUS had a significantly higher sensitivity in staging lung cancer when compared to EBUS-TBNA alone with the additional benefit of diagnosing mediastinal adenopathy (68). In an analysis of 276 patients, Torii et al. found that adding EUS-B-FNA to EBUS-TBNA increased the diagnostic yield from 72.6% to 75.9% (28).
Occult metastases in radiographically normal mediastinum appear to occur more frequently in patients with central, solid, and adenocarcinomatous tumors (69). However, adenocarcinomatous histology was also associated with a higher prevalence of false negative results when examined by endosonography (70). Despite this, combined EBUS with EUS/EUS-B has a role in detecting occult disease. Our institution evaluated 161 patients with CT and PET scans showing normal lymph nodes in the mediastinum, hilum, lobar, and sublobar regions (44). After endoscopic staging using EBUS and EUS, 13% of patients were found to have nodal disease. Of those with positive nodal disease, 28% of patients had occult N1 disease, 61% had occult N2 disease, and 9% had adrenal involvement. A retrospective study of 279 patients by Kim et al. found a 29.7% prevalence of occult metastases with 38.6% of those cases being detected by EBUS with EUS-B (69). Shin et al. found a sensitivity of 47% using EBUS with or without EUS-B to detect occult malignancy (70). In a recent study, EUS when performed after EBUS was found to be more useful in upstaging disease from N1 to multilevel N2/N3 disease rather than from N0 to N1/N2 (71). Because combined endoscopy can detect N3 disease that may otherwise be missed on surgical mediastinoscopy, there is an important role for endosonography in staging of occult disease.
Despite being a combination of two procedures, depending on user familiarity and technique, combining EBUS/EUS only slightly increases the procedure time (27,36).
Combined procedure compared to surgical approach
The combined approach has better results compared to surgical mediastinoscopy. A prospective controlled trial comparing combined EBUS/EUS with mediastinoscopy showed sensitivity, negative predictive value, and diagnostic accuracy of 91%, 96%, and 97% respectively in EBUS/EUS as compared to 79%, 90%, and 93% in mediastinoscopy (18). Another prospective trial designed by Liberman et al. found that combined EBUS/EUS diagnosed N2/N3/M1 disease in 14% of patients who had negative findings using standard mediastinoscopy (67). These findings are supported by a multicenter randomized study that found the sensitivity of endosonographic staging of N2/N3 disease superior to that of surgical staging (72).
Through the cumulative benefits of a combined procedure, surgery can be avoided. Hwangbo et al. showed increased diagnostic accuracy and overall sensitivity when EUS-B-FNA was added to EBUS (36). While EBUS had a sensitivity of 84.4% with diagnostic accuracy of 95.1%, adding EUS-B-FNA increased the overall sensitivity to 91.1% and diagnostic accuracy to 97.2%. By combining the two procedures, mediastinoscopy was avoidable in 41 of 150 patients.
Current guidelines recommend surgical staging if there is a high index of suspicion of metastases despite negative endosonography. However, there is newer data that suggests that endoscopy may replace the need of surgical confirmation. A recently published study by Bousema et al. evaluated the outcomes of lung tumor resection following systematic endosonography with or without confirmatory mediastinoscopy (73). While there was an unforeseen N2 rate that was detected by mediastinoscopy, it did not exceed the study’s noninferiority boundary, suggesting that confirmatory mediastinoscopy can be omitted in the event of a negative systemic endosonographic evaluation of the patient. In a patient with a radiographically normal mediastinum, when biopsies obtained with combined endoscopic procedure are negative, there is a trend towards omission of surgical exploration of the mediastinum (24,44). Through its wide range of access from mediastinal lymph nodes to subdiaphragmatic structures, EBUS/EUS/EUS-B can potentially replace surgical staging with NSCLC.
Limitations
Despite the wide range of nodes expected to be covered by EUS/EUS-B, not all lesions can be biopsied due to technical reasons. Authors Hwangbo et al. found that there was difficulty in accessing station 5, possibly due to the narrow sonographic angle of the linear probe with EUS-B (36). Other limitations to EUS-B when compared to EUS include fixed needle angle, and limited push against the esophageal wall (27,36). Both EUS/EUS-B have limited access to the anterior nodes and structures when compared to standard EBUS.
In patients who have already received chemo-radiotherapy, samples obtained by EUS/EUS-B may not be as reliable. In a study by von Bartheld et al., restaging the mediastinum with EUS-FNA alone after chemo-radiation had a sensitivity of 44% and a false negative rate of 58% (74). Combined EBUS/EUS has a better sensitivity of 76% and specificity of 100% in restaging after neoadjuvant treatment (75,76). However, in a study evaluating combined EUS/EUS-B in restaging the mediastinum, 17% of patients with negative biopsy by endoscopy had metastatic disease when re-examined by transcervical extended mediastinal lymphadenectomy (TEMLA) (76,77). These differences in re-staging by endoscopy may be due to post-inflammatory changes such as adhesions and fibrosis (78). The current ESGE/ERS/ESTS guidelines suggest that restaging after neoadjuvant chemo-radiation may be performed by either endoscopic method. However, if there is no detection of persistent disease, surgical mediastinal staging may be indicated prior to radical surgery (grade C recommendation) (14).
Physician familiarity and experience with EUS/EUS-B limits extensive use of this procedure. Diagnostic yield is dependent on the operator’s level of skill. There is currently no standardized training for pulmonologists interested in using EUS/EUS-B. Ng et al. found that with supervision and training, experienced bronchoscopists can perform EBUS/EUS-B with relative ease (79). After 3 consecutive cases with an Endoscopic Ultrasound Assessment Tool (EUSAT) score of 50 or higher, the provider was determined competent in performing EUS-B-FNA as part of their usual practice without added supervision. American Thoracic Society (ATS), ERS, and ACCP recommend 40 procedures for initial competency and afterwards, 20 procedures to maintain competency. Though gastrointestinal guidelines require 150 EUS-FNA of the pancreas before achieving competency, this number may be lower in EUS-B-FNA due to easier maneuverability of the bronchoscope as well as easier accessibility of the mediastinal lymph nodes (67,79-82).
Conclusions
The development of endosonographic techniques including EBUS and EUS/EUS-B have led to the replacement of mediastinoscopy as the initial test in staging of NSCLC. Though each procedure has proven useful in identifying malignancy, they have limitations in target accessibility. Combining EBUS and EUS/EUS-B provides thorough coverage of the mediastinum and even includes structures inferior to the diaphragm. There is an additive value and the resulting sensitivity and negative predictive value are higher than that of a single procedure. EUS has played a large role in decreasing the need for surgical interventions for diagnostic sampling. The selection of EUS or EUS-B in conjunction with EBUS would depend on the user’s familiarity and comfort level with the techniques. For the average bronchoscopist, the EUS-B offers the advantage of shorter endoscope length as it is the same device used for EBUS. However, in the hands of an experienced provider, EUS can provide better visualization of the lesion due to differences in probe ultrasound and access to more distal structures such as the right adrenal gland and right lobe of the liver. Regardless of which procedure is added to EBUS, these combined techniques have repeatedly shown a benefit in complete staging, time to diagnosis, patient comfort, and overall cost.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathan Kurman and Bryan S. Benn) for the series “Diagnostic & Therapeutic Bronchoscopy” published in AME Medical Journal. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-114/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-114/coif). The series “Diagnostic & Therapeutic Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Global Burden of Disease 2019 Cancer Collaboration; Kocarnik JM, Compton K, et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol 2022;8:420-44.
- De Wever W, Ceyssens S, Mortelmans L, et al. Additional value of PET-CT in the staging of lung cancer: comparison with CT alone, PET alone and visual correlation of PET and CT. Eur Radiol 2007;17:23-32. [Crossref] [PubMed]
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178S-201S.
- Dales RE, Stark RM, Raman S. Computed tomography to stage lung cancer. Approaching a controversy using meta-analysis. Am Rev Respir Dis 1990;141:1096-101. [Crossref] [PubMed]
- Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500-7. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ojha B, et al. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg 2005;80:1207-13; discussion 1213-4. [Crossref] [PubMed]
- McLoud TC, Bourgouin PM, Greenberg RW, et al. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology 1992;182:319-23. [Crossref] [PubMed]
- Tournoy KG, Maddens S, Gosselin R, et al. Integrated FDG-PET/CT does not make invasive staging of the intrathoracic lymph nodes in non-small cell lung cancer redundant: a prospective study. Thorax 2007;62:696-701. [Crossref] [PubMed]
- Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:137S-46S. [Crossref] [PubMed]
- Silvestri GA, Hoffman B, Reed CE. One from column A: choosing between CT, positron emission tomography, endoscopic ultrasound with fine-needle aspiration, transbronchial needle aspiration, thoracoscopy, mediastinoscopy, and mediastinotomy for staging lung cancer. Chest 2003;123:333-5. [Crossref] [PubMed]
- Toloza EM, Harpole L, Detterbeck F, et al. Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:157S-66S. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:545-59. [Crossref] [PubMed]
- Hegde PV, Liberman M. Mediastinal Staging: Endosonographic Ultrasound Lymph Node Biopsy or Mediastinoscopy. Thorac Surg Clin 2016;26:243-9. [Crossref] [PubMed]
- Ernsguided transbronchial needle aspiration of hilar lymph nodes for diagnosing and t A, Eberhardt R, Krasnik M, et al. Efficacy of endobronchial ultrasound-staging cancer. J Thorac Oncol 2009;4:947-50.
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Silvestri GA, Bevill BT, Huang J, et al. An Evaluation of Diagnostic Yield From Bronchoscopy: The Impact of Clinical/Radiographic Factors, Procedure Type, and Degree of Suspicion for Cancer. Chest 2020;157:1656-64. [Crossref] [PubMed]
- Kuijvenhoven JC, Leoncini F, Crombag LC, et al. Endobronchial Ultrasound for the Diagnosis of Centrally Located Lung Tumors: A Systematic Review and Meta-Analysis. Respiration 2020;99:441-50. [Crossref] [PubMed]
- Iliaz S, Caglayan B, Bulutay P, et al. Rapid On-site Evaluation and Final Cytologic Diagnoses Correlation During Endobronchial Ultrasonography. J Bronchology Interv Pulmonol 2022;29:191-7. [Crossref] [PubMed]
- Mangiavillano B, Sosa-Valencia L, Deprez P, et al. Tissue acquisition and pancreatic masses: Which needle and which acquisition technique should be used? Endosc Int Open 2020;8:E1315-20. [Crossref] [PubMed]
- Crombag LMMJ, Szlubowski A, Stigt JA, et al. EUS-B-FNA vs conventional EUS-FNA for left adrenal gland analysis in lung cancer patients. Lung Cancer 2017;108:38-44. [Crossref] [PubMed]
- Szlubowski A, Soja J, Kocon P, et al. A comparison of the combined ultrasound of the mediastinum by use of a single ultrasound bronchoscope versus ultrasound bronchoscope plus ultrasound gastroscope in lung cancer staging: a prospective trial. Interact Cardiovasc Thorac Surg 2012;15:442-6; discussion 446. [Crossref] [PubMed]
- Mangiavillano B, Spatola F, Facciorusso A, et al. Transesophageal endoscopic ultrasound in the diagnosis of the lung masses: a multicenter experience with fine-needle aspiration and fine-needle biopsy needles. Eur J Gastroenterol Hepatol 2022;34:757-762. [Crossref] [PubMed]
- Baker JJ, Solanki PH, Schenk DA, et al. Transbronchial fine needle aspiration of the mediastinum. Importance of lymphocytes as an indicator of specimen adequacy. Acta Cytol 1990;34:517-23. [PubMed]
- Hwangbo B, Lee HS, Lee GK, et al. Transoesophageal needle aspiration using a convex probe ultrasonic bronchoscope. Respirology 2009;14:843-9. [Crossref] [PubMed]
- Torii A, Oki M, Yamada A, et al. EUS-B-FNA Enhances the Diagnostic Yield of EBUS Bronchoscope for Intrathoracic Lesions. Lung 2022;200:643-8. [Crossref] [PubMed]
- Khoury J, Adir Y, Schneer S, et al. Awake endoscopic (esophageal) ultrasound using the endobronchial scope (EUS-B) for patients with high risk for sedation. Respir Med Res 2022;82:100944. [Crossref] [PubMed]
- Kang HJ, Hwangbo B, Lee GK, et al. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax 2014;69:261-8. [Crossref] [PubMed]
- Annema JT, Veseliç M, Rabe KF. EUS-guided FNA of centrally located lung tumours following a non-diagnostic bronchoscopy. Lung Cancer 2005;48:357-61; discussion 363-4. [Crossref] [PubMed]
- Varadarajulu S, Hoffman BJ, Hawes RH, et al. EUS-guided FNA of lung masses adjacent to or abutting the esophagus after unrevealing CT-guided biopsy or bronchoscopy. Gastrointest Endosc 2004;60:293-7. [Crossref] [PubMed]
- Fritscher-Ravens A, Soehendra N, Schirrow L, et al. Role of transesophageal endosonography-guided fine-needle aspiration in the diagnosis of lung cancer. Chest 2000;117:339-45. [Crossref] [PubMed]
- Korevaar DA, Crombag LM, Cohen JF, et al. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med 2016;4:960-8. [Crossref] [PubMed]
- Hwangbo B, Kang HJ. Author's response to 'Complete (EBUS and EUS) endosonographic staging of lung cancer'. Thorax 2014;69:675-6. [Crossref] [PubMed]
- Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010;138:795-802. [Crossref] [PubMed]
- Liberman M, Duranceau A, Grunenwald E, et al. Initial experience with a new technique of endoscopic and ultrasonographic access for biopsy of para-aortic (station 6) mediastinal lymph nodes without traversing the aorta. J Thorac Cardiovasc Surg 2012;144:787-92; discussion 792-3. [Crossref] [PubMed]
- Liberman M, Duranceau A, Grunenwald E, et al. New technique performed by using EUS access for biopsy of para-aortic (station 6) mediastinal lymph nodes without traversing the aorta (with video). Gastrointest Endosc 2011;73:1048-51. [Crossref] [PubMed]
- Pravachan V.C. Hegde, Eric Goudie, Moishe Liberman. Endosonographic Lymph Node Staging by Combined Endobronchial Ultrasound (EBUS) and Endoscopic Ultrasound: Technique and Technical Tricks, Operative Techniques in Thoracic and Cardiovascular Surgery, Volume 23, Issue 3, 2018, Pages 136-150, ISSN 1522-2942,
10.1053/j.optechstcvs.2018.12.003 .10.1053/j.optechstcvs.2018.12.003 - Obiols C, Call S, Rami-Porta R, et al. Survival of patients with unsuspected pN2 non-small cell lung cancer after an accurate preoperative mediastinal staging. Ann Thorac Surg 2014;97:957-64. [Crossref] [PubMed]
- Colella S, Vilmann P, Konge L, et al. Endoscopic ultrasound in the diagnosis and staging of lung cancer. Endosc Ultrasound 2014;3:205-12. [Crossref] [PubMed]
- Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [Crossref] [PubMed]
- Assisi D, Gallina FT, Forcella D, et al. Transesophageal Endoscopic Ultrasound Fine Needle Biopsy for the Diagnosis of Mediastinal Masses: A Retrospective Real-World Analysis. J Clin Med 2022;11:5469. [Crossref] [PubMed]
- Hegde P, Molina JC, Thivierge-Southidara M, et al. Combined Endosonographic Mediastinal Lymph Node Staging in Positron Emission Tomography and Computed Tomography Node-Negative Non-Small-Cell Lung Cancer in High-Risk Patients. Semin Thorac Cardiovasc Surg 2020;32:162-8. [Crossref] [PubMed]
- Giri S, Harindranath S, Angadi S, et al. Efficacy and safety of endosonography-guided transvascular needle aspiration of thoracic and abdominal lesions: A systematic review and meta-analysis. J Clin Ultrasound 2023;51:723-30. [Crossref] [PubMed]
- Kazakov J, Hegde P, Tahiri M, et al. Endobronchial and Endoscopic Ultrasound-Guided Transvascular Biopsy of Mediastinal, Hilar, and Lung Lesions. Ann Thorac Surg 2017;103:951-5. [Crossref] [PubMed]
- Molina JC, Chaudry F, Menezes V, et al. Transvascular endosonographic-guided needle biopsy of intrathoracic lesions. J Thorac Cardiovasc Surg 2020;159:2057-65. [Crossref] [PubMed]
- von Bartheld MB, Rabe KF, Annema JT. Transaortic EUS-guided FNA in the diagnosis of lung tumors and lymph nodes. Gastrointest Endosc 2009;69:345-9. [Crossref] [PubMed]
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [Crossref] [PubMed]
- LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc 2004;59:475-81. [Crossref] [PubMed]
- Hegde P, Liberman M. Echo-endoscopic lymph node staging in lung cancer: an endoscopic alternative. Expert Rev Anticancer Ther 2015;15:1063-73. [Crossref] [PubMed]
- Patil R, Ona MA, Papafragkakis C, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of adrenal lesions. Ann Gastroenterol 2016;29:307-11. [Crossref] [PubMed]
- Lee MJ, Hahn PF, Papanicolaou N, et al. Benign and malignant adrenal masses: CT distinction with attenuation coefficients, size, and observer analysis. Radiology 1991;179:415-8. [Crossref] [PubMed]
- Pennathur A, Luketich JD, Heron DE, et al. Stereotactic radiosurgery for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg 2009;137:597-604. [Crossref] [PubMed]
- Xiang H, Han J, Ridley WE, et al. Tadpole adrenal and seagull adrenal: Normal anatomic appearance. J Med Imaging Radiat Oncol 2018;62:112. [Crossref] [PubMed]
- Pais FM, Shah RA, Vempilly JJ, et al. Transesophageal approach to lung, adrenal biopsy and fiducial placement using endoscopic ultrasonography (EUS): An interventional pulmonology experience. Initial experience of the UCSF-FRETOC (fresno tracheobronchial & oesophageal center) study group. Respir Med 2018;141:52-5. [Crossref] [PubMed]
- Schuurbiers OC, Tournoy KG, Schoppers HJ, et al. EUS-FNA for the detection of left adrenal metastasis in patients with lung cancer. Lung Cancer 2011;73:310-5. [Crossref] [PubMed]
- Puri R, Thandassery RB, Choudhary NS, et al. Endoscopic ultrasound-guided fine-needle aspiration of the adrenal glands: analysis of 21 patients. Clin Endosc 2015;48:165-70. [Crossref] [PubMed]
- Nasir BS, Edwards M, Tiffault V, et al. Transesophageal pulmonary nodule biopsy using endoscopic ultrasonography. J Thorac Cardiovasc Surg 2014;148:850-5; discussion 855. [Crossref] [PubMed]
- Bugalho A, Ferreira D, Eberhardt R, et al. Diagnostic value of endobronchial and endoscopic ultrasound-guided fine needle aspiration for accessible lung cancer lesions after non-diagnostic conventional techniques: a prospective study. BMC Cancer 2013;13:130. [Crossref] [PubMed]
- Larsen SS, Krasnik M, Vilmann P, et al. Endoscopic ultrasound guided biopsy of mediastinal lesions has a major impact on patient management. Thorax 2002;57:98-103. [Crossref] [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [Crossref] [PubMed]
- Tournoy KG, De Ryck F, Vanwalleghem LR, et al. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am J Respir Crit Care Med 2008;177:531-5. [Crossref] [PubMed]
- Yasuda I, Kato T, Asano F, et al. Mediastinal lymph node staging in potentially resectable non-small cell lung cancer: a prospective comparison of CT and EUS/EUS-FNA. Respiration 2009;78:423-31. [Crossref] [PubMed]
- Fernández-Esparrach G, Ginès A, Belda J, et al. Transesophageal ultrasound-guided fine needle aspiration improves mediastinal staging in patients with non-small cell lung cancer and normal mediastinum on computed tomography. Lung Cancer 2006;54:35-40. [Crossref] [PubMed]
- Herth FJ, Krasnik M, Kahn N, et al. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010;138:790-4. [Crossref] [PubMed]
- Liberman M, Sampalis J, Duranceau A, et al. Endosonographic mediastinal lymph node staging of lung cancer. Chest 2014;146:389-97. [Crossref] [PubMed]
- Dhooria S, Aggarwal AN, Gupta D, et al. Utility and Safety of Endoscopic Ultrasound With Bronchoscope-Guided Fine-Needle Aspiration in Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Respir Care 2015;60:1040-50. [Crossref] [PubMed]
- Kim BG, Cho JH, Shin SH, et al. Diagnostic Performance of Endosonography to Detect Mediastinal Lymph Node Metastasis in Patients with Radiological N1 Non-Small Cell Lung Cancer. Cancer Res Treat 2023;55:832-40. [Crossref] [PubMed]
- Shin SH, Jeong BH, Jhun BW, et al. The utility of endosonography for mediastinal staging of non-small cell lung cancer in patients with radiological N0 disease. Lung Cancer 2020;139:151-6. [Crossref] [PubMed]
- Crombag LMM, Dooms C, Stigt JA, et al. Systematic and combined endosonographic staging of lung cancer (SCORE study). Eur Respir J 2019;53:1800800. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [Crossref] [PubMed]
- Bousema JE, Dijkgraaf MGW, van der Heijden EHFM, et al. Endosonography With or Without Confirmatory Mediastinoscopy for Resectable Lung Cancer: A Randomized Clinical Trial. J Clin Oncol 2023;41:3805-15. [Crossref] [PubMed]
- von Bartheld MB, Versteegh MI, Braun J, et al. Transesophageal ultrasound-guided fine-needle aspiration for the mediastinal restaging of non-small cell lung cancer. J Thorac Oncol 2011;6:1510-5. [Crossref] [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Szlubowski A, Herth FJ, Soja J, et al. Endobronchial ultrasound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy--a prospective study. Eur J Cardiothorac Surg 2010;37:1180-4. [Crossref] [PubMed]
- Szlubowski A, Zieliński M, Soja J, et al. Accurate and safe mediastinal restaging by combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscope. Eur J Cardiothorac Surg 2014;46:262-6. [Crossref] [PubMed]
- Muthu V, Sehgal IS, Dhooria S, et al. Efficacy of Endosonographic Procedures in Mediastinal Restaging of Lung Cancer After Neoadjuvant Therapy: A Systematic Review and Diagnostic Accuracy Meta-Analysis. Chest 2018;154:99-109. [Crossref] [PubMed]
- Ng J, Chan HP, Kee A, et al. Transitioning to Combined EBUS EUS-B FNA for Experienced EBUS Bronchoscopist. Diagnostics (Basel) 2021;11:1021. [Crossref] [PubMed]
- Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy 2012;44:190-206. [Crossref] [PubMed]
- Konge L, Annema J, Vilmann P, et al. Transesophageal ultrasonography for lung cancer staging: learning curves of pulmonologists. J Thorac Oncol 2013;8:1402-8. [Crossref] [PubMed]
- Vilmann P, Săftoiu A. Endoscopic ultrasound-guided fine needle aspiration biopsy: equipment and technique. J Gastroenterol Hepatol 2006;21:1646-55. [Crossref] [PubMed]
Cite this article as: Ko J, Hegde P. Added value of endoscopic ultrasound to endobronchial ultrasound in non-small cell lung cancer staging. AME Med J 2024;9:2.