Donation after circulatory death: a narrative review of current controversies, attitudes, and the evolving role of regional perfusion technology
Introduction
There are two distinct pathways for deceased organ donation today: donation after brain death (DBD) and donation after circulatory death (DCD). DCD was previously known as non-heart-beating donation (NHBD), donation after cardiac death and more recently as donation after the circulatory determination of death (DCDD). DCD is the term in most common use today (1). Patients who might be considered as DCD organ donors are divided into groups known as the Maastricht categories. These were most recently updated in 2013 (Table 1) (2).
Table 1
| Category | Clinical scenario | Location | Circulatory death uDCD or cDCD | Warm ischaemic time |
|---|---|---|---|---|
| Ia | Found dead unwitnessed | Out of hospital | Uncontrolled | Approximate calculation |
| Ib | Found dead unwitnessed | In-hospital | Uncontrolled | Approximate calculation |
| IIa | Witnessed cardiac arrest | Out of hospital | Uncontrolled | Approximate calculation |
| IIb | Witnessed cardiac arrest | In-hospital | Uncontrolled | Approximate calculation |
| III | Withdrawal of life-sustaining-therapy | In-hospital | Controlled | Known exactly |
| IV | Cardiac arrest during or after criteria for BSD completed | In-hospital | Controlled/uncontrolled | Known exactly |
uDCD: permanent and irreversible circulatory death determined on the basis that patient cannot be resuscitated—failed resuscitation. cDCD: permanent and irreversible circulatory death determined on the basis that patient will not be resuscitated—do not attempt resuscitation order in place. Maastricht category V: legislation in 18 jurisdictions allows organ donation after MAiD, ODE or organ donation after VAD (3). DCD, donation after circulatory death; uDCD, uncontrolled DCD; cDCD, controlled DCD; BSD, brain death also termed brainstem death; MAiD, medical assistance in dying; ODE, organ donation after euthanasia; VAD, voluntary assisted dying.
DCD is a significant growth area in organ donation. In 2019, DCD donations accounted for 20% of all donated organs in the European Union (EU) (4). Similarly in 2021, it accounted for 30% of donated organs in the USA (5). Despite this increase, DCD has complex ethical, cultural and legal aspects which have limited its introduction worldwide, despite expert society endorsement (6-16).
Organ utilisation rates (OURs) are lower with DCD. In the UK, an average of 3.6 organs are transplanted per DBD donation, compared with 2.1 organs after a DCD donation (17). Similar figures are reported in Switzerland (3.5 vs. 2.3) (18).
DCD has limitations from an organ quality perspective. DCD organs suffer a period of hypoperfusion known as the warm ischaemic time (WIT). Hypoperfusion is most damaging at systolic blood pressures below 50 mmHg: the onset of the functional WIT (fWIT) (Table 2).
Table 2
| Acronym | Complete term | Definition |
|---|---|---|
| A-NRP | Abdominal-normothermic regional perfusion | Synonyms: nECMO, normothermic (regional) extracorporeal membrane oxygenation; ANOR, abdominal normothermic oxygenation & recirculation; EISOR, extracorporeal interval support for organ retrieval |
| TA-NRP | Thoraco-abdominal NRP | A partial circulation which excludes the cerebral circulation only through ligation and division of carotid and subclavian arteries |
| WIT | Warm ischaemic time | Withdrawal time + primary warm ischaemic time. Synonyms: donor WIT, total WIT |
| Withdrawal time | Withdrawal of life sustaining therapies until circulatory arrest. Synonym: agonal phase | |
| Primary warm ischaemic time | Circulatory arrest until in-situ perfusion of organs. Synonyms: asystolic phase, first WIT | |
| fWIT | Functional warm ischaemic time | Begins—systolic BP <50 mmHg. Ends—in-situ perfusion of organs |
| WLST | Withdrawal of life-sustaining therapies | Changing goals of therapy to comfort and palliative measures. Reflects discontinuation of therapy: stopping mechanical ventilation, inotropes, dialysis, cardiovascular mechanical supports ECMO, VADs, IABP |
| cDCD | Controlled donation after circulatory death | Permanent and irreversible circulatory death determined on the basis that patient will not be resuscitated—do not attempt resuscitation order in place |
| uDCD | Uncontrolled donation after circulatory death | Permanent and irreversible circulatory death determined on the basis that patient cannot be resuscitated—failed resuscitation |
| ECD | Expanded criteria donor (20) | Based on a 70% greater risk of developing graft failure vs donor kidneys aged between 10 and 39 years. Defined as: any donor aged ≥60 years or any donor aged 50–59 years plus two of: CVA death; creatinine >133 µmol/L; hypertension |
| DGF | Delayed graft function | The need for dialysis in the 7 days after transplantation |
| PNF | Primary non-function (21) | Renal: dialysis dependence or creatinine clearance ≤20 mL/minute at 3 months post-transplant. Hepatic: an aggravated form of reperfusion injury resulting in irreversible graft failure without detectable technical or immunological problems. Need for retransplant from between 72 hours to 10 days following transplant or death. Seen in 3% DBD and 9% DCD hepatic transplants |
| ITBL/IC | Ischaemic type biliary lesions; ischaemic cholangiopathy | Presents with cholestasis or progressive ischemic biliary injury, majority occur within 1 year of transplant: bile duct necrosis, bile leakage, biloma, bile duct fibrosis, bile duct stenosis. Incidence in DCD typically 16–29% |
NRP, normothermic regional perfusion; BP, blood pressure; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device; IABP, intra-aortic balloon pump; CVA, cerebrovascular accident; DBD, donation after brain death; DCD, donation after circulatory death.
As a result, DCD organs are prone to early graft dysfunction, increased reintervention rates and frequently have inferior outcomes when compared with DBD organs. Livers are predisposed to vascular stenosis, ischaemic cholangiopathy and consequently, increased costs (22). Delayed graft function (DGF) in kidneys is significant and patients remain longer in hospital (23).
Despite warm ischaemic injury, outcomes from DCD lung transplantation are encouraging with equal primary graft dysfunction, acute rejection rates and mortality reported (24,25). These results are supported by a systematic review of 17 studies from Europe, the USA and Australia. This said, airway anastomotic complications were twice as likely to occur in DCD lungs (DCD 8–29% vs. DBD 4–14%) (26). While DCD cardiac transplantation also has been successful, either in-situ or ex-situ perfusion is an essential prerequisite to implantation. Studies of DCD simultaneous pancreas-kidney transplants have demonstrated almost equal outcomes to DBD pancreas transplant. However, it is likely that many more pancreas transplants could be performed if the maximum WIT could be extended (27,28).
Where the time from withdrawal of life-sustaining therapies (WLST) to cardiac arrest (known as the withdrawal time) exceeds that set by the transplant team, the process of DCD is stood down. This is reported in up to 25% of DCD attempts in the USA and 40% of DCD attempts in the UK (5,17) (Table 3). An attempted but non-progressing DCD can discourage those involved from pursuing the process in the future. Terms including “waste” and “loss of valuable organs” have been used by donor families. Healthcare professionals have used the expression “a second loss” to describe the experience of families when the process is stood down (29).
Table 3
| Organ | Australian | British | Canadian | USA |
|---|---|---|---|---|
| Kidney, minutes | 60 (240 in selected patients) | 180 | 120 | 45–60 |
| Liver, minutes | 30 | 20 | 30 | 30 |
| Lungs, minutes | 90 | 60 | 60 | 60 |
| Pancreas, minutes | 60 | 30 (pancreatic islet cells 60 minutes) | 60 | 60 |
In contrast to DBD, estimates of flow and perfusion are not possible during the rapid retrieval operation typical of DCD. Time pressures during the retrieval operation may predispose to organ injury. In context, a reticence to transplant organs where there are legitimate concerns about the degree of ischaemic or mechanical injury is understandable.
The objectives of this review are multiple. Firstly, we aim to examine areas of discourse and obstacles to the wider adoption of DCD with particular emphasis placed on terminology and language. We examine reasons why DCD has been slow to gain momentum and present two areas where international variation is evident: the time of death and the case for antemortem heparin.
Secondly, we present the options for organ preservation in DCD: in-situ cold perfusion (ISP), super-rapid retrieval (SRR) and the most recent evolution of practice—normothermic regional perfusion (NRP). We provide a summary of consensus guidelines and a synopsis of evidence on the use of NRP.
Thirdly, we briefly discuss the preservation of DCD organs using ex-situ technologies. Finally, we discuss ethics particular to NRP, namely antemortem cannulation, post-mortem cerebral isolation and the prevention of collateral perfusion of the brain.
Similar publications individually cover at least one of these four facets in detail (30-32). The target audience are healthcare professionals, from those with a superficial knowledge of DCD to those in Intensive Care Medicine or Transplantation and people in a position to implement change within their own organisation. We present this article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-23-65/rc).
Methods
We performed a database search as outlined in Table 4. Two reviewers assessed each article. The articles selected and summarised in Tables 5-7 deal with outcomes primarily from liver and kidney transplantation. The references section of each article was accessed and relevant articles examined for inclusion. General outcome measures of organ transplantation were retrieved and summarised, including DGF, primary non-function (PNF), OUR together with graft and patient survival. Delayed post-transplant complications of liver transplantation such as cholangiopathy, ischaemic type biliary lesions (ITBLs) and vascular complications are included. Websites of learned bodies and organ donor organisations were accessed where we sought position and consensus statements. These learned bodies included the Society of Critical Care Medicine (USA), the Intensive Care Society (UK), the Australian and New Zealand Intensive Care Society (ANZICS) and other international transplantation societies.
Table 4
| Items | Specification |
|---|---|
| Date of search | 1st March 2023 |
| Databases and other sources searched | PubMed, Embase, and the Cochrane Library. Online databases searched included UpToDate and Dynamed. Websites of learned bodies and organ donor organisations |
| Search terms | Transplantation (MeSH); Tissue and Organ procurement (MeSH); Donation after circulatory death; Ethics and organ donation; Normothermic regional Perfusion, regional perfusion; Normothermic extracorporeal membranous oxygenation; Extracorporeal interval support for organ retrieval; Delayed Graft function, primary non function; Organ utilisation rates; Heparin and Organ Donation; Controlled donation after circulatory death; Uncontrolled donation after circulatory death |
| Timeframe | Between January 2000 and June 2023 |
| Inclusion and exclusion criteria | Inclusion criteria: (I) systematic reviews, randomised controlled trials, comparator studies, case series, reviews, consensus statements, editorial and expert opinion pieces; (II) English language. Exclusion criteria: (I) non-standard terminology; (II) sample size precluded analysis of NRP impact; (III) paper published in language other than English |
| Selection process | Reviewers J.O.R. and A.G., reviewed each article independently and reached consensus on inclusion onto tables |
NRP, normothermic regional perfusion.
Table 5
| Author (reference); article type | Principle outcome measures reported |
|---|---|
| Artiles Medina A (33); Systematic Review | In-situ preservation techniques in cDCD and uDCD in renal transplants |
| Studies limited by heterogeneity, retrospective, and small size | |
| • NRP associated with decreased DGF vs. ISP or TBC | |
| OR =0.36 (95% CI: 0.25–0.54) | |
| Hessheimer AJ (34); Consensus Statement | All reported studies demonstrate overall improved outcomes for liver transplantation where NRP utilized |
| Bansal S (35); Systematic Review Abstract publication | DGF following NRP-DCD renal transplant vs. DBD |
| • Average DGF DCD-NRP: renal transplants 23% | |
| • Average DGF DBD: renal transplants 28% | |
| [OR =0.47 (95% CI: 0.27–0.81); P<0.01] | |
| De Beule J (36); Systematic Review | Hepatic: NRP—risk of EAD ↓ 56% vs. in situ perfusion; NRP—risk of IC ↓ 75% vs. in situ perfusion; NRP—anastomotic strictures ↓ 65% vs. in situ perfusion |
| Renal: DGF not different between DBD and NRP-DCD | |
| Pancreas: limited cases but 100% graft survival at 1 year | |
| van de Leemkolk FEM (37); Systematic Review | Heterogeneity of studies limits interpretation |
| • Most single centre | |
| • None double blinded or randomized, NRP protocols vary | |
| • Temperature of NRP 27–37 degrees | |
| • Flow targets 1.7–4.0 lpm | |
| Conclusions | |
| (I) Results show that NRP is feasible and safe | |
| (II) OUR: NRP > ISP > TBC | |
| (III) “In France, Italy and Norway, abdominal NRP has become the standard procurement procedure for DCD donors mandated by the health authorities or preferred routine in several regions in the UK and Spain” | |
| (IV) “Function and outcomes after kidney and liver transplantation using NRP appear superior to non-abdominal NRP DCD when comparing data to large cohorts described elsewhere” |
↓, decrease or decreased. cDCD, controlled donation after circulatory death; uDCD, uncontrolled donation after circulatory death; NRP, normothermic regional perfusion; DGF, delayed graft function; ISP, in-situ cold perfusion; TBC, total body cooling; OR, odds ratio; CI, confidence interval; DCD, donation after circulatory death; DBD, donation after brain death; EAD, early allograft dysfunction; IC, ischaemic cholangiopathy; lpm, litres per minute; OUR, organ utilisation rate.
Table 6
| Controlled DCD: author, country/region, (reference) | Comparators | Hepatic | Renal | Outcomes |
|---|---|---|---|---|
| Oniscu GC, UK, 2023, multicentre, (38) | A-NRP cDCD vs. RR cDCD | 94 vs. 1,376 | 210 vs. 5,744 | OUR improved: 3.3 vs. 2.6; 1-year survival better in hepatic group; DGF ↓ 35% renal, ↑ GFR at 1 year 6.3 mL/min/1.73 m2 |
| Bekki Y, USA, 2023, (39) | TA-NRP vs. RR DCD | 34 vs. 136 | 62 | ↑ liver OUR: 70.6% vs. 39%; ↑ kidney OUR: 94% vs. 78%; ↓ creatinine at 1 year |
| Croome KP, USA, 2023, (40) | A-NRP | 11 | 20 | Liver OUR 78% (11/14); 0% PNF; 0% IC on follow up; 10% renal DGF (2/20) |
| Padilla M, Spain, 2021, multicentre, (41) | A-NRP cDCD vs. RR cDCD | – | 770 vs. 770 | RR DGF ↑ OR 1.97; RR graft loss at 1 year ↑ OR 1.77; RR creatinine at 1 year ↑ |
| Savier E, France, 2020, multicentre, (42) | A-NRP cDCD vs. DBD | 50 vs. 100 | – | Transaminases, early allograft dysfunction, 1- & 2-year graft survival, ITBL, cholangiopathy, similar between groups |
| Mori G, Italy, 2020, (43) | A-NRP cDCD vs. DBD | – | 7 vs. 28 | GFR at 1-year DGF equal, DBD 62 mL/min, DCD NRP 54 mL/min |
| Hessheimer AJ, Spain, 2019, multicentre, (44) | A-NRP cDCD (n=152) vs. (N=218) SRR | 95 (OUR 64%) vs. 117 (OUR 57%) | – | NRP: better PNF, ITBL, early allograft dysfunction, graft survival at 1 year; “superior outcome with NRP vs. SRR” |
| Watson CJE, UK, 2019, multicentre, (45) | cDCD-NRP vs. cDCD-SRR | 47 vs. 187 | – | Significantly ↓. Biliary strictures 7% vs. 27%; ischemic cholangiopathy & 3-month graft loss 2% vs. 10% |
| Rodríguez-Sanjuán JC, Spain, 2019, (46) | A-NRP cDCD vs. DBD | 11 vs. 51 | Equal outcomes, low risk of PNF and IC with cDCD-NRP | |
| Pearson R, UK, 2021, abstract publication, (47) | A-NRP cDCD vs. DCD-SRR | – | 29 vs. 200 | ↓ DGF 14%—NRP vs. 35%—SRR. Improved GFR for 3 years post-transplant |
| Ruiz P, Spain, 2019, (48) | A-NRP cDCD (N=57) | 46 vs. OUR 81% | – | No IC; no graft loss |
| Foss S, Norway, 2018, (49) | A NRP cDCD | – | 14 | DGF 7% |
| Mowlem E, UK, 2017, abstract publication, (50) | A-NRP cDCD vs. SRR cDCD | 20 vs. 40 | – | NRP less graft damage: ↓ ALT levels, ischemic cholangiopathy 0% |
| Miñambres E, Spain, 2017, (51) | A NRP cDCD | 11 | 37 | DGF 27%. 6 double lungs and 1 pancreas all transplanted |
| Giadrosich BE, Spain, 2018, (52) | A- NRP cDCD vs. SRR cDCD | – | 17 vs. 46 | 17% DGF vs. 62% RR |
| Oniscu GC, UK, 2014, (53) | A NRP cDCD (N=16) | 8 | 24 | DGF kidney 20%, IC 0%, low peak ALT |
| Rojas-Peña A, USA, 2014, (54) | A-NRP cDCD (N=37) | 13 | 48 | DGF 31% kidneys. Hepatic grafts PNF & IC 14% |
| Farney AC, USA, 2011, (55) | cDCD with EISOR vs. SRR cDCD | – | 19 vs. 115 | DGF 21% cDCD-EISOR, 54% RR |
| Lee CY, Taiwan, 2005, (56) | Mixed cDCD & uDCD | – | 31 | DGF 41% NRP; DGF 27% DBD; graft survival equal at 5 years |
| Magliocca JF, USA, 2005, (57) | cDCD (N=15) | 5 | 24 | DGD kidneys 8%; liver PNF 0% |
| Gravel MT, USA, 2004, (58) | cDCD-NRP | – | 19 | DGF 11% |
↑, increase or increased; ↓, decrease or decreased. NRP, normothermic regional perfusion; DCD, donation after circulatory death; A-NRP, abdominal NRP; cDCD, controlled DCD; RR, rapid retrieval; TA-NRP, thoraco-abdominal NRP; DBD, donation after brain death; SRR, super rapid retrieval; EISOR, extracorporeal interval support for organ retrieval; uDCD, uncontrolled DCD; OUR, organ utilisation rate; DGF, delayed graft function; GFR, glomerular filtration rate; PNF, primary non-function; OR, odds ratio; ITBL, ischaemic type biliary lesions; IC, ischaemic cholangiopathy; ALT, alanine aminotransferase; vs., versus comparator group.
Table 7
| Uncontrolled DCD: author, country, (reference) | Comparators | Hepatic | Renal | Outcome |
|---|---|---|---|---|
| Antoine C, France, 2020, multicentre (59)* | uDCD-NRP vs. uDCD-ISP. No flow <30 min; fWIT <150 min | – | 234 vs. 265 | NRP ↓, PNF 6% vs. 9%, OR 1.83; poor graft function at 1 year 9.8% vs. 19%, OR 2.6 |
| Molina M, Spain, 2019, (60) | uDCD-NRP vs. DBD | – | 237 vs. 237 | DGF 72% vs. 46%. Equal GFR at 10 years |
| Delsuc C, France, 2018, (61) | uDCD-NRP vs. uDCD-ISP | 32 vs. 32 | – | Decreased LOHS & DGF, 84% vs. 72% with NRP. Better GFR up to 2 years post-transplant |
| Demiselle J, France, 2016, (62) | uDCD-NRP vs. uDCD-ISP | – | 19 NRP vs. 31 ISP | DGF 53% vs. 81%, NRP better graft function for up to 2 years |
| Reznik ON, Russia, 2013, (63) | uDCD-ECMO subnormothermic perfusion leucodepletion & thrombolytics vs. DBD | – | 44 vs. 87 | 50% DGF in DCD, equal creatinine at 3/12, equal 1 year graft survival |
| Valero R, Spain, 2000, (64) | uDCD ISP vs. TBC vs. NRP | – | 40 vs. 8 vs. 8 | DGF 12.5% with NRP ↓↓ vs. others |
*, one centre used NRP in 2008 but all 15 centres converted based on results. ↓, decrease or decreased; ↓↓, significantly decreased. NRP, normothermic regional perfusion; DCD, donation after circulatory death; uDCD, uncontrolled DCD; ISP, in-situ cold perfusion; fWIT, functional warm ischaemic time; DBD, donation after brain death; TBC, total body cooling; PNF, primary non function; OR, odds ratio; DGF, delayed graft function; GFR, glomerular filtration rate; LOHS, length of hospital stay; vs., versus, comparator group.
Language, attitudes and controversies in DCD
Language and attitudes around DCD
The role of language and terminology is an important and perhaps underappreciated area; the language surrounding DCD in particular must be identified and managed. In one study, healthcare professionals experienced in both DBD and DCD were presented with a series of terms and words with positive and negative connotations, significantly more negative words were ascribed to the practice of DCD (65). Terms such as “controlled” and “uncontrolled” populate DCD literature, yet who would wish to be involved in an “uncontrolled process”? In DCD, the terms “controlled” and “uncontrolled” are used, not as a measure of clinical coordination, but rather to reflect applicable time constraints for authorisation or consent and the logistics of organising retrieval teams.
Futility, in reference to further medical treatment, is inextricably linked to the process of DCD however the term itself should ideally be avoided. The word futile may be misinterpreted by relatives to reflect a lack of empathy on the part of the medical professional.
Care is never “withdrawn”, the focus of care is redirected towards palliative measures to allow, insofar as is possible, natural death to occur. Irregular or partially obstructed breathing, frequently referred to as “agonal”, is evident is some patients. This does not necessarily mean patient distress and this word is best avoided.
Healthcare professionals must be careful in how they present the case for DCD, how the topic is introduced and the timing of such an approach. Professional bodies recommend a period of separation between informing families that end-of-life care is appropriate and discussions about the potential for DCD. Where NRP is contemplated, terminology such as “extracorporeal membrane oxygenation”, “resuscitation” and “reanimation”, must be considered carefully before use. Others favour the terms “in-situ tissue perfusion” or “dynamic in-situ organ assessment” (66).
There are aspects of DCD which may negatively influence healthcare professionals’ attitudes. Maastricht “category V” is included in many classifications. This is organ donation following medical assistance in dying (MAiD), organ donation after euthanasia (ODE) or voluntary assisted dying (VAD). While most pathways are characterised by the requirement for patient-initiated request, first-person consent and periods of reflection, many healthcare professionals express unease with these processes (3).
Some transplant professionals argue that controlled DCD (cDCD) may represent a shorter path for intensive care clinicians to follow rather than spending time to determine if brain death will ensue. This is termed “substitution”, and while a valid concern, it is rarely the case. In cDCD, the determination of medical futility is based on clinical examination, radiological investigations and electroencephalographic investigations. Given the scope of investigations required, a significant time may have elapsed before a decision to WLST is reached. An international collaborative of clinicians recommends delaying WLST for a period of 72 hours to improve prognostication. This time will determine the trajectory of the patient’s neurological condition (67).
Defining death and what is reversible
It is important to consider opinions from those opposed to DCD; their criticisms can only serve to develop a more comprehensive understanding for all. The German Medical Association guided by the German Ethics Council have repeatedly rejected the concept of DCD (68). They state that while the diagnosis of brain death is definitive, they argue that DCD involves unacceptable uncertainty.
In Maastricht categories I and II [uncontrolled DCD (uDCD)]: when does irreversible become completely irreversible? While informed by protocols and expert opinion there cannot be complete certainty that resuscitation becomes irreversible at even 30, 60 or 90 minutes.
In Maastricht category III (cDCD): when can medically administered therapies to sustain life be deemed inappropriate with absolute certainty? After how many minutes of circulatory arrest is it appropriate to diagnose death and proceed with organ donation? And finally, could the decision to withdraw life sustaining therapies be influenced in any way by the potential for organ donation?
The uncontrolled categories of DCD (Maastricht categories I and II) have been criticised because of contention around the appropriate duration of resuscitation. The European Resuscitation Council suggest cardiopulmonary resuscitation (CPR) for 90 minutes following arrest where pulmonary embolism is suspected and thrombolytics administered (17). In the era of extracorporeal CPR (eCPR), emergency percutaneous coronary intervention and advanced resuscitation techniques, clinicians must continuously appraise the evidence to avoid the premature termination of resuscitation (29). In later publications, the same authors and others argue that in the setting of failed eCPR, that eCPR and uDCD protocols may coexist and complement each other (18,30).
In cDCD (Maastricht category III), the potential for premature WLST is a frequently cited as an area of concern. Decisions to redirect therapy towards end-of-life care are not made lightly. The UK Donation Ethics Committee recommends two doctors, one a consultant and a second senior doctor, make the decision that further active treatment is no longer of benefit to the patient (69). The Irish National Guideline stipulates that this decision is made by at least two intensive care consultants and a third consultant whose speciality is neurological, such as a neurologist, neurosurgeon, or stroke physician (6). Therefore, at least three consultants and frequently four must reach consensus before consideration is given to DCD. Clinicians involved in caring for these patients at the end of life are not transplant professionals and there is separation between of end-of-life care and transplantation.
The German Medical Association emphasises the importance of confidence and trusts in the medical profession. Although this is certainly important, medical professionals also have a responsibility to pursue change where it may yield benefit for patients. Clinicians are best placed to collate and examine the evidence and in partnership with legal professionals, propose changes to the law if needed (68,70). Decisions regarding the acceptability of DCD in any jurisdiction must be led by medical professionals and have substantive input from ethics, law, and lay members.
In several ways DCD is a straightforward process, and with over 50 years of experience it is a proven concept. The knowledge surrounding the diagnosis of death is explicit and variability in the conduct of DCD is not desirable, hence the importance of national and international consensus documents. Confidence and trust in the system of organ donation is promoted by clarity of the processes involved and the knowledge that the process has been subject to scrutiny (71).
The diagnosis and time of death
The diagnosis of death must satisfy the criterion of permanence. An accepted definition from an expert panel is:
“Death is the permanent loss of capacity for consciousness and all brainstem functions. This may result from permanent cessation of circulation or catastrophic brain injury. In the context of death determination, ‘permanent’ refers to loss of function that cannot resume spontaneously and will not be restored through intervention” (36).
One criticism of DCD is the variability in time periods before death is diagnosed. This inconsistency arises due to concerns around the potential for autoresuscitation (Table 8).
Table 8
| Country | Observation period, minutes | Type of DCD program | Source |
|---|---|---|---|
| USA | 2–5 | uDCD & cDCD | Gries et al. SCCM (10) |
| Canada | 5 | cDCD | Shemie et al. (8) |
| UK | 5 | cDCD | NHSBT (7) |
| Australia | 5 | cDCD | ANZICS 2021, (16) |
| Austria | 10 | uDCD & cDCD | Lomero M et al. (72) |
| Portugal | 10 | uDCD | Lomero M et al. (72) |
| Italy | 20 | uDCD & cDCD | Lomero M et al. (72); Mori G et al. (43) |
| Russia | 30 | uDCD | Lomero M et al. (72) |
DCD, donation after circulatory death; uDCD, uncontrolled DCD; cDCD, controlled DCD; SCCM, Society of Critical Care Medicine; NHSBT, National Health Service: Blood and Transplant; ANZICS, Australian and New Zealand Intensive Care Society.
The word “irreversible” frequently appears within definitions of death. “Irreversible” may mean:
- The loss of function that cannot be restored now or at any time in the future, or
- The loss of function that cannot be restored by those present at the time, or
- The loss of function that will not resume and will not be restored.
In the context of DCD, once the period where autoresuscitation is possible has passed, and provided that no attempts will be made to restore circulation as in (III), then the permanence criterion has been satisfied and death may be diagnosed.
ANZICS describe death as a “process” where cellular and organ functions progressively cease:
“The determination and certification of death indicate that an irrevocable point in the dying process has been reached. The precise time of death is somewhat arbitrary and represents a societal consensus that is informed by biological understanding” (16).
While similar to the statement from the expert panel review (above), the word “irrevocable” in context defines the time beyond which autoresuscitation is possible.
In 2023, the Canadian “brain-based definition of death” and the criteria for its determination were published. The scope of the guideline is to include all potential organ donors who will undergo death determination by circulatory criteria or by neurological criteria. The “brain-based diagnosis of death” unifies circulatory death and brain death on the basis of an absent circulation causing the permanent cessation of brain functions. The unified brain-based criteria stipulate that death may result from cessation of blood circulation to the brain after circulatory arrest and/or from devastating brain injury (73,74).
This has significant implications in terms of in-situ establishment of a regional circuit of warmed blood post-mortem, in other words, the permissibility of NRP. In Australian law, circulatory death is diagnosed after the circulation stops and if a circulation is started (including via a regional perfusion circuit) then the patient is no longer dead legally, therefore NRP is not permitted at present (75).
While there are legal, ethical, cultural and religious aspects to the diagnosis of death, there is an inextricable link between circulation and neuronal function. After 20 seconds of circulatory arrest, the electroencephalogram (EEG) becomes isoelectric, the biological events that follow are subject to the potential for resuscitation and the intentions of bystanders. Once the time where the potential for autoresuscitation has elapsed, then neuronal function may only be re-established by creating a circulation. Therefore, the exact time that death is diagnosed is dependent on the clinical setting (76).
cDCD
Autoresuscitation following WLST is extremely rare. The largest prospective observational study involved 631 patients. Clinically reported resumption of cardiac activity (an arterial pressure of at least 5 mmHg), respiratory movement, or both confirmed by waveform analysis occurred in 1% of the sample (5 patients). The longest duration of pulselessness before resumption of cardiac activity was 4 minutes 20 seconds (77).
In an updated systematic review by the same investigators, the measured incidence of autoresuscitation in cases of WLST with or without DCD was 1.8% (19 of 1,049 patients). The principal recommendation from this review was that a 5-minute observation period is sufficient for cDCD, a view supported by most clinicians (Maastricht category III) (78).
uDCD
Autoresuscitation has been described in situations of failed resuscitation after up to 10 minutes. Therefore, in Maastricht categories I and II, it is reasonable to ensure a period of 10 minutes of circulatory arrest has elapsed before death is diagnosed (77).
The case for antemortem heparin
The administration of antemortem heparin in DCD must be considered in context. A medication which provides no direct benefit to the donor patient may be considered “not in their best interests”. However, “best interests” may extend beyond the patient’s medical needs:
“Best interests must also include their social, emotional, cultural and religious interests, so if a patient wished to donate their organs, it would be in their best interests to ensure that the organs are transplanted in the best possible condition” (79).
The UK Donation Ethics Committee called for further work to determine if the use of ante-mortem heparin should be revisited, objections were legal in nature. It stated that there is no ethical barrier, provided an individualised risk assessment is completed (69). In the UK, antemortem heparin is administered to Maastricht category IV donor patients (80).
The Australian Organ Donation and Tissue Authority national protocol and the National Health and Medical Research Council support antemortem interventions providing there is no legal impediment, and state that they support:
“Administering heparin [e.g., 25,000 units (or 300 U/kg)] to prevent small-vessel thrombosis —[however] if there is any concern than heparin may foreshorten the patient’s life, the heparin can be given when the patient is apnoeic.” (16).
In 2013, the American Thoracic Society, the International Society for Heart and Lung Transplantation and the Society of Critical Care Medicine deemed the administration of antemortem heparin ethically acceptable once the risks and potential benefits were disclosed (10). The American Association of Thoracic Surgery 2023 expert consensus document states (in relation to adult cardiac transplantation) that in DCD:
“Administration of intravenous heparin at the point of the WLST is the current standard of care. There are no reported cases of heparin administered at this time hastening death” (81).
In summary, although there is no benefit to the potential donor patient, the principle of non-maleficence must also be considered. Antemortem heparin decreases the incidence of PNF of liver grafts and the rate of vascular thrombosis in pancreatic grafts (82,83). Beneficial effects in other organs are less clear, but may include improved machine perfusion (MP) indices and less glomerular microthrombi in renal transplant and less pulmonary thrombi in lung transplantation.
The evolution of organ preservation
Organ preservation techniques used in DCD
Four techniques are used to minimise the ischaemic injury suffered by organs after circulatory death.
ISP
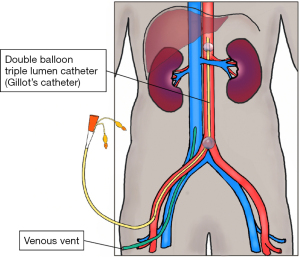
Cold perfusate is flushed into the femoral artery though a double balloon triple lumen catheter. This localises perfusion to the abdominal organs, a venous vent drains the effluent. Rates of flow are reduced as the venous effluent becomes clear. In practice, this technique is limited to uDCD (Figure 1).
SRR
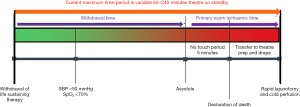
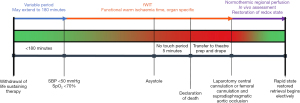
The flow diagram outlines the process from WLST until laparotomy (Figure 2). At the end of a limited period of organ ischaemia, this technique involves a rapid laparotomy, isolation of the inferior vena cava (IVC) and aorta and immediate cold flushing before recovering the organs.
NRP
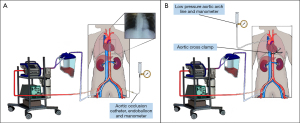
In the context of DCD, NRP is an enhanced therapy, delivered post-mortem which ameliorates the effects of ischaemia on donated organs (Figures 3,4). Regional perfusion may be limited to the abdominal-NRP (A-NRP), or to thoraco-abdominal-NRP (TA-NRP). The focus of this paper is the use of A-NRP in cDCD (Maastricht categories III and IV).
NRP has the potential to increase the time-period from WLST until organ donation begins by several hours. If the timer starts with the onset of fWIT, and NRP is used, then it is likely that less stand-downs will occur. The number of organs retrieved per patient are higher than with standard DCD (3.3 vs. 2.6) (38). While on NRP, macroscopic appearances and trends in biochemical parameters provide reassurance of post-transplant function.
Ex-situ MP technologies
Normothermic MP (NMP) and hypothermic oxygenated perfusion (HOPE) modalities enable ex-situ organ assessment. These machines are portable and facilitate organ transport with ongoing artificial nutrients and oxygen perfusion. Several trials have demonstrated comparable outcomes for A-NRP and NMP liver transplants where graft dysfunction and OUR are examined (84,85). In heart transplantation, the direct procurement protocol and MP (DPP-MP) strategy has yielded equal outcomes to a matched cohort of DBD heart donors. In the USA, 80% of DCD heart donors are retrieved by DPP-MP (86), this is also the current standard in Australia and the UK (87).
While ex-vivo technologies are ethically less problematic, expense is significant; the cost of disposables for the NRP circuit is $4,000 USD, while the Heart OCS™ (Transmedics, Inc., Andover, MA, USA) averages $46,000 USD (88).
NRP
Evidence for the use of NRP
Protocols, process and outcomes of NRP in the context of DCD are widely published (22,33-36,67,72,89-95). Online webinar series are available from the European Society of Organ Transplantation and the Organ Donation Alliance (12,96). The use of NRP is evidence-based and supported by individuals and expert groups in numerous reviews and consensus statements (Table 5).
The importance and contribution of perfusion repair is supported by an international group from the USA, the UK, Canada, Australia and other European centres (67).
“The value of perfusion repair… is established by this consensus statement to recommend that a protocol of cDCD utilise either in-situ or ex-situ perfusion consistent with the practice of each country conducting cDCD”.
The evidence for the use of NRP in DCD is supported by review and consensus articles (Table 5) in both cDCD (Table 6) and uDCD (Table 7). NRP allows time for the close inspection and viability assessments of perfused abdominal organs. It permits the elective recovery of organs and reduces the potential for organ damage as a consequence of the SRR technique.
While the economic case for the adoption of NRP has been proven in the context of hepatic transplantation, ultimately, some commentators suggest the economic case for NRP could be championed by the renal allograft experience (41,79,94).
Benefits of NRP
Reduced DGF in renal transplants
Earlier improved renal graft function will decrease hospital stay and the sustained improvement in glomerular filtration rate will lead to longer graft function in recipients.
Improved outcomes from hepatic transplantation
Previously, rates of ischaemic cholangiopathy seen in DCD have been major deterrents. NRP ameliorates the damaging effects of warm ischaemia and outcomes seen with DCD-NRP approximate those seen with DBD. Consequently, the Spanish Liver Transplant Society and the International Liver Transplant Society both recommend NRP in DCD liver transplant (34,97).
DCD lung transplantation
There has been concern that the adoption of A-NRP may impede lung retrieval, or will lead to a reduction in the number of lungs retrieval in DCD. While technically more complex, A-NRP and lung retrieval in DCD may be combined; a dual hypothermic thoracic and normothermic abdominal approach may be the technique of choice. Reports from Spain have demonstrated similar outcomes when compared with DBD donors (24,38,51,98).
Where lung retrieval is contemplated using TA-NRP, anterograde perfusion will not be possible until the heart begins beating (67). Despite this, demonstrated outcomes have been excellent albeit with small numbers (n=26) (99).
Essential components of an NRP protocol
Multiple NRP protocols are available online, which vary depending on their country of origin (72,95,100-102). The development of an institutional and national protocol for NRP is a difficult process. The agreement of key stakeholders in transplant surgery, transplant medicine, intensive care medicine, legal professional bodies and the broader medical community is an essential step. One or more bodies may be charged with the application of professional standards and accreditation based on guidelines, quality assurance, audit and governance.
The separation and professional independence of intensive care medicine and transplantation is of paramount importance. Specific aspects of intensive care unit (ICU) management include the location of end-of-life care, the WLST, acceptable antemortem interventions, diagnosing death and preparation of the patient following death. In all protocols there is a specific focus on ischaemic times, with a strong emphasis on go and no-go timepoints.
Adopting NRP is likely to result in significant baseline capital expenditure. There are additional ongoing costs of funding rotas, recruitment and training. In the UK, specialist training and certification is required for the role of Advanced Perfusion and Organ Preservation Specialist (APOPS), an individual central to the success of NRP (101).
Specific ethical considerations in NRP
A significant number of DCD attempts do not progress to organ donation as patients may not die within the timeframes outlined. End-of-life care may be a significant stress for medical and nursing staff, and, where time constraints are involved in the context of organ donation, an attempted but non-progressing organ donation can discourage future attempts (29). NRP significantly decreases these time constraints. While there are several contentious areas, we focus on two: patient cannulation and the potential for residual flow where the cerebral circulation is not adequately excluded.
Postmortem cannulation
Patient cannulation for the purposes of A-NRP may be achieved post-mortem via femoral or central vessels. In A-NRP, the aorta and IVC are exposed and cannulated at the point at which they bifurcate in the lower abdomen. In TA-NRP, the thoracic aorta and right atrium are cannulated in the chest.
Antemortem cannulation
There is a balance between what antemortem cannulation can offer and the risks it poses to the patient. International practice varies considerably regarding the permissibility of cannulation in the setting of cDCD. Some jurisdictions are completely opposed, while others such as Spain allow complete cannulation for A-NRP antemortem. In other jurisdictions, a more nuanced approach is adopted: vessel localising cannulas are placed in the femoral vessels antemortem, these are then upsized after death. It could be argued that there is merit and dignity for the potential organ donor patient where successful postmortem femoral cannulation is guaranteed, therefore avoiding a rapid cut-down or pressurised laparotomy. This then allows a slow and careful dissection for organ retrieval.
Peripheral cannulation: antemortem vessel localisation for percutaneous femoral cannulation
Placing two sheaths using local anaesthesia, ultrasound and micropuncture techniques will minimise pain and discomfort. The sheaths are then covered with a sterile dressing. These cannulas do not require patient movement or X-ray confirmation and may be removed with minimal discomfort if DCD is stood down.
In 2019, an ethical evaluation on the use of A-NRP in cDCD was conducted by the Norwegian Institute of Public Health. It was their assessment:
“that the antemortem insertion of thin cannulas into the femoral arteries and veins does not deviate from good patient care or differ in ethical terms from practices adopted to support DBD.” (102).
Others argue:
“there is a certain amount of intellectual strain involved in thinking antemortem interventions [are] motivated by a concern for the best interests of the donor”;
“While antemortem interventions may promote an autonomous wish to donate organs, critics worry that this may come at the cost of causing deontological harm.” (103).
Echoing the case for antemortem heparin, discussions return to an interpretation of patient’s best interests. The patient’s beliefs, values, and social, cultural and altruistic interests should be considered alongside their clinical interests (79,104,105).
Preventing reconstitution of the cerebral circulation
In the period following death, the principle of permanence mandates that there must be no flow of blood outside the limited NRP circuit. This must be absolute. Escape of blood outside of the oxygenated circuit and into the cerebral circulation risks the potential for nociception (106). Pressures within the abdominal aorta during A-NRP are 38–65 mmHg, while pressures above the aortic occlusion balloon are 5 cmH2O (3 mmHg) or less.
A second area of concern in A-NRP is the potential escape of oxygenated blood through small collateral circulations. Theoretically, blood might travel via a series of anatomical connections to reach the central nervous system (CNS).
Whether the escape of blood from an abdominal NRP circuit could result in a return of spontaneous cerebral and cardiac activity (ROSCCA) is important. One systematic review addressed this question in 484 donor patients in 12 case series (58). In 472 patients the aorta was occluded by an aortic balloon and in 12 cases by aortic cross-clamp, the authors concluded:
“There is no evidence to suggest that ROSCCA may occur following the institution of A-NRP in DCDs where the proximal aorta has been occluded.”
In TA-NRP, despite ligation of the carotid and subclavian arteries, small amounts of blood flow have been demonstrated to drain from the distal ligated ends of these vessels. The recommendation is that the cranial end of each vessel is opened and allowed to drain freely.
The diagnosis of death is contingent upon the absence of cerebral circulation and the prohibition of any intervention which could potentially cause a resumption of cerebral blood flow. Concerns that NRP could invalidate the diagnosis of death have been raised by the American College of Physicians. In particular, concerns have been raised in cases of TA-NRP where the heart may be restarted once the cerebral circulation has been disconnected (107). Some argue that the surgical ligation and exclusion of the carotid and vertebral arteries makes the surgeon complicit in the patient’s death. Other authors refute these assertions and argue, where the patient has been diagnosed with cardiocirculatory death, this diagnosis stands once the cerebral circulation has been isolated:
“the act of clamping the cerebral circulation, occurs after death and so it does not bring on death”;
“Restoring the function of the heart through an ECMO circuit is not autoresuscitation or resuscitation of the person”… [it provides];
“mechanically assisted circulation to maintain perfusion and oxygenation of organs for transplantation” (73,74,106,108-114).
Commentators on the brain-based definition of death add that the circulation of the brain is the foremost concern and state:
“Provided brain function has ceased before initiation of NRP, ligation, occlusion, or transection [of the aortic arch vessels] do not induce cessation of brain function they maintain it.” (114).
The prohibition of manoeuvres which might cause a resumption of cerebral blood flow are the bedrock upon which the diagnosis of death by circulatory criteria is based, i.e., the principle of permanence. Yet, if circulation is re-established by NRP, could this diagnosis of death be retrospectively invalidated? The brain-based concept of death effectively answers this question in stating that the relevant circulation which must have ceased permanently is the circulation to the brain:
“If NRP is effectively restricted to ensure no circulation to the brain, thereby preventing brain perfusion and function, NRP fulfils the requirements of donor death determination and respects the dead donor rule.” (74).
A-NRP is seen by many authors as an option less likely to cause collateral perfusion than TA-NRP because the thoracic aorta, internal thoracic and intercostal arteries are excluded. Surgical ligation of lumbar vessels and the ligation of the femoral vessels exclude superficial epigastric flows (58).
In A-NRP where an endoclamp or aortic occlusion balloon is used, the wiring lumen of the endovascular balloon catheter provides a monitoring port for pressure. This lumen opens outside of the NRP circuit and may be opened to atmospheric pressure. In open surgical cannulation, the aorta is most frequently occluded by an external aortic cross-clamp. A line placed in the supradiaphragmatic aorta above this cross-clamp is then left open to atmospheric pressure. Both “flow diversion” lines act as the lowest pressure point in the body, expected pressures above the aortic balloon or external aortic clamp are less than 5 cmH2O or 0–3 mmHg (Figure 4) (80,106).
In both situations, first—failure of the external or internal aortic cross-clamp and second—collateral circulations, an aortic arch pressure less than 5 cmH2O provides a central sump or low pressure point in the circulation. Where doubt exists about the integrity of the cross-clamp, or excessive blood is emitted from the flow diversion line, A-NRP must be discontinued and cold perfusion commenced. These recommendations are consistent with, and satisfy the requirements of, the UK National protocol for A-NRP (80).
Conclusions
DCD can be a challenging process, but organ donation has helped many families gain solace from knowing that the altruistic gift of their loved one is helping others. While protocolised therapy has its place, the adoption of NRP must be in the context of the team, the protocol, the equipment, the oversight, audit and governance. We see NRP as a significant area of improvement in DCD. In many respects it reduces stress for all involved, reducing time constraints in the ICU, reducing the pressure on surgeons to rapidly retrieve the organs and reducing the risk of graft dysfunction in recipients.
In France, Italy and Norway, A-NRP has become the standard organ retrieval procedure for DCD donors mandated by the health authorities. It is the preferred procedure in several regions in the UK and Spain. The function and outcomes after kidney and liver transplantation using NRP appear superior to non-A-NRP DCD.
The future of organ donation may comprise a suite of technologies to include in-situ perfusion-NRP and ex-situ perfusion devices such as the OCS™ (Transmedics) device for cardiac support, mobile ex-situ lung perfusion and the OrganOx (Oxford, UK) machine for ex-situ liver perfusion. While each of these technologies has something to offer, perhaps NRP offers most in terms of ameliorating ischaemic injuries to several organs simultaneously. Although TA-NRP has hurdles to overcome before gaining widespread acceptance, the adoption of A-NRP is an easier route in the short-term.
The authors acknowledge the limitations of this review, there are a notable lack of prospective randomised trials concerning the use of NRP and other ex-situ technologies. Previous meta-analysis has commented on the heterogenous nature of many studies. Each included study was at the discretion of the authors and may not represent a thorough quality analysis of each paper. While we acknowledge the obstacles, the evidence supports the more widespread adoption of NRP.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-65/prf
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-23-65/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-65/coif). J.O.R. is a clinical lead in organ donation and a national clinical lead for Donation after Circulatory Death (DCD). He is member of the advisory board of the Organ Donation Transplant Ireland (ODTI) – The National Organ Donation and Transplant Advisory Group (NODTAG), this is not a paid role. He provides clinical support to donor coordinators within the national organ procurement service. Standard on-call rates of pay apply to this role. J.O.R. serves as an unpaid editorial board member of AME Medical Journal from January 2023 to December 2024. A.G. is a clinical lead in organ donation and works closely with Organ Donation and Transplant Ireland (ODTI). Part of his role consultant’s salary is supported by ODTI. He provides advisory to the ODTI in areas such as of strategy, education, and planning. He provides clinical support to consultants in other hospitals in areas relating to organ donation and transplant. He is a member of the advisory board of the Organ Donation Transplant Ireland – The National Organ Donation and Transplant Advisory Group (NODTAG). He is the national lead for the potential donor audit. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- da Graca B, Borries T, Polk H, et al. Ethical Issues in Donation following Circulatory Death: A Scoping Review Examining Changes over Time from 1993 to 2022. AJOB Empir Bioeth 2023;14:237-77. [Crossref] [PubMed]
- Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int 2016;29:749-59. [Crossref] [PubMed]
- Mulder J, Sonneveld H, Van Raemdonck D, et al. Practice and challenges for organ donation after medical assistance in dying: A scoping review including the results of the first international roundtable in 2021. Am J Transplant 2022;22:2759-80. [Crossref] [PubMed]
- Vanholder R, Domínguez-Gil B, Busic M, et al. Organ donation and transplantation: a multi-stakeholder call to action. Nat Rev Nephrol 2021;17:554-68. [Crossref] [PubMed]
- Seshadri A, Cuschieri J, Kaups KL, et al. Organ donation in the surgical ICU: an American Association for the Surgery of Trauma Critical Care Committee clinical consensus document. Trauma Surg Acute Care Open 2023;8:e001107. [Crossref] [PubMed]
- O’Rourke J, Marsh B, Dwyer R. The Intensive Care Society of Ireland Guidelines on donation after circulatory death. 2016. Available online: https://jficmi.anaesthesia.ie/wp-content/uploads/2017/05/DCD-ICSI-Guideline.pdf
- Andrews PA, Burnapp L, Manas D, et al. Summary of the British Transplantation Society guidelines for transplantation from donors after deceased circulatory death. Transplantation 2014;97:265-70. [Crossref] [PubMed]
- Shemie SD, Baker AJ, Knoll G, et al. National recommendations for donation after cardiocirculatory death in Canada: Donation after cardiocirculatory death in Canada. CMAJ 2006;175:S1. [Crossref] [PubMed]
- Weiss MJ, Hornby L, Rochwerg B, et al. Canadian Guidelines for Controlled Pediatric Donation After Circulatory Determination of Death-Summary Report. Pediatr Crit Care Med 2017;18:1035-46. [Crossref] [PubMed]
- Gries CJ, White DB, Truog RD, et al. An official American Thoracic Society/International Society for Heart and Lung Transplantation/Society of Critical Care Medicine/Association of Organ and Procurement Organizations/United Network of Organ Sharing Statement: ethical and policy considerations in organ donation after circulatory determination of death. Am J Respir Crit Care Med 2013;188:103-9. [Crossref] [PubMed]
- United Network for Organ Sharing USA. Critical Pathway for Donation After Cardiac Death. Available online: https://unos.org/wp-content/uploads/Critical_Pathway_DCD_Donor.pdf
- Organ Donation and Transplantation Alliance. Conversation Series: DCD in Heart Transplant - The Case for Normothermic Regional Perfusion 2022. Available online: https://www.youtube.com/watch?v=DLX_QpDoUD4&t=1s
- Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant 2009;9:2004-11. [Crossref] [PubMed]
- Office of the Chief Health Officer: New South Wales Government. GL2021_012: Organ Donation After Circulatory Death; 2021.
- Australian Government Organ and Tissue Authority. Best Practice Guideline for Donation after Circulatory Determination of Death (DCDD) in Australia. Australia: Australian Government Organ and Tissue Authority; 2021.
- The Australian and New Zealand Intensive Care Society. The ANZICS Statement on Death and Organ Donation (Edition 4.1). Edition 4.1 ed. Melbourne: ANZICS; 2021.
- Manara AR, Murphy PG, O'Callaghan G. Donation after circulatory death. Br J Anaesth 2012;108:i108-21. [Crossref] [PubMed]
- Elmer A, Rohrer ML, Benden C, et al. Organ donation after circulatory death as compared with organ donation after brain death in Switzerland - an observational study. Swiss Med Wkly 2022;152:w30139. [Crossref] [PubMed]
- European Directorate for the Quality of Medicines and Healthcare. Guide to the quality and safety of organs for transplantation. In: Council of Europe, editor. 8 ed: The Council of Europe; 2022.
- Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant 2003;3:114-25. [Crossref] [PubMed]
- Bernat JL, D'Alessandro AM, Port FK, et al. Report of a National Conference on Donation after cardiac death. Am J Transplant 2006;6:281-91. [Crossref] [PubMed]
- van der Hilst CS, Ijtsma AJ, Bottema JT, et al. The price of donation after cardiac death in liver transplantation: a prospective cost-effectiveness study. Transpl Int 2013;26:411-8. [Crossref] [PubMed]
- O'Connell C, Ferede AA, Williams Y, et al. Kidney donation after circulatory death; an opportunity to expand the donor pool. European Urology. Supplement 2019; [Crossref]
- Campo-Cañaveral de la Cruz JL, Miñambres E, Coll E, et al. Outcomes of lung and liver transplantation after simultaneous recovery using abdominal normothermic regional perfusion in donors after the circulatory determination of death versus donors after brain death. Am J Transplant 2023;23:996-1008. [Crossref] [PubMed]
- Bobba CM, Whitson BA, Henn MC, et al. Trends in Donation After Circulatory Death in Lung Transplantation in the United States: Impact Of Era. Transpl Int 2022;35:10172. [Crossref] [PubMed]
- Zhou J, Chen B, Liao H, et al. The Comparable Efficacy of Lung Donation After Circulatory Death and Brain Death: A Systematic Review and Meta-analysis. Transplantation 2019;103:2624-33. [Crossref] [PubMed]
- Owen R, Counter C, Tingle S, et al. O004 Impact of normothermic regional perfusion on recipient outcomes after simultaneous pancreas and kidney transplantation – a UK analysis from the NHSBT Pancreas Advisory Group. Br J Surg 2023; [Crossref]
- Qureshi MS, Callaghan CJ, Bradley JA, et al. Outcomes of simultaneous pancreas-kidney transplantation from brain-dead and controlled circulatory death donors. Br J Surg 2012;99:831-8. [Crossref] [PubMed]
- Taylor LJ, Buffington A, Scalea JR, et al. Harms of unsuccessful donation after circulatory death: An exploratory study. Am J Transplant 2018;18:402-9. [Crossref] [PubMed]
- Smith M, Dominguez-Gil B, Greer DM, et al. Organ donation after circulatory death: current status and future potential. Intensive Care Med 2019;45:310-21. [Crossref] [PubMed]
- Lepoittevin M, Giraud S, Kerforne T, et al. How to improve results after DCD (donation after circulation death). Presse Med 2022;51:104143. [Crossref] [PubMed]
- De Carlis R, Centonze L, Migliorini M, et al. Abdominal normothermic regional perfusion in donation after circulatory death: organ viability or organ preservation? European Journal of Transplantation 2023;2:113-20. [Crossref]
- Artiles Medina A, Burgos Revilla FJ, Álvarez Nadal M, et al. Comparison of in situ preservation techniques for kidneys from donors after circulatory death: a systematic review and meta-analysis. Transl Androl Urol 2021;10:3286-99. [Crossref] [PubMed]
- Hessheimer AJ, Gastaca M, Miñambres E, et al. Donation after circulatory death liver transplantation: consensus statements from the Spanish Liver Transplantation Society. Transpl Int 2020;33:902-16. [Crossref] [PubMed]
- Bansal S, O’Neill S, Oniscu G. Systematic Review of Outcomes Following Transplantation with Organs Recovered from cDCD Donors Using Normothermic Regional Perfusion. Transplant International 2017;30:351-69.
- De Beule J, Vandendriessche K, Pengel LHM, et al. A systematic review and meta-analyses of regional perfusion in donation after circulatory death solid organ transplantation. Transpl Int 2021;34:2046-60. [Crossref] [PubMed]
- van de Leemkolk FEM, Schurink IJ, Dekkers OM, et al. Abdominal Normothermic Regional Perfusion in Donation After Circulatory Death: A Systematic Review and Critical Appraisal. Transplantation 2020;104:1776-91. [Crossref] [PubMed]
- Oniscu GC, Mehew J, Butler AJ, et al. Improved Organ Utilization and Better Transplant Outcomes With In Situ Normothermic Regional Perfusion in Controlled Donation After Circulatory Death. Transplantation 2023;107:438-48. [Crossref] [PubMed]
- Bekki Y, Croome KP, Myers B, et al. Normothermic Regional Perfusion Can Improve Both Utilization and Outcomes in DCD Liver, Kidney, and Pancreas Transplantation. Transplant Direct 2023;9:e1450. [Crossref] [PubMed]
- Croome KP, Brown TE, Mabrey RL, et al. Development of a portable abdominal normothermic regional perfusion (A-NRP) program in the United States. Liver Transpl 2023;29:1282-91. [Crossref] [PubMed]
- Padilla M, Coll E, Fernández-Pérez C, et al. Improved short-term outcomes of kidney transplants in controlled donation after the circulatory determination of death with the use of normothermic regional perfusion. Am J Transplant 2021;21:3618-28. [Crossref] [PubMed]
- Savier E, Lim C, Rayar M, et al. Favorable Outcomes of Liver Transplantation from Controlled Circulatory Death Donors Using Normothermic Regional Perfusion Compared to Brain Death Donors. Transplantation 2020;104:1943-51. [Crossref] [PubMed]
- Mori G, Solazzo A, Tonelli L, et al. Comparison Between Kidney Transplantation After Circulatory Death and After Brain Death: A Monocentric Retrospective Study After 1 Year of Follow-up. Transplant Proc 2020;52:1536-8. [Crossref] [PubMed]
- Hessheimer AJ, Coll E, Torres F, et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol 2019;70:658-65. [Crossref] [PubMed]
- Watson CJE, Hunt F, Messer S, et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant 2019;19:1745-58. [Crossref] [PubMed]
- Rodríguez-Sanjuán JC, Ruiz N, Miñambres E, et al. Liver Transplant From Controlled Cardiac Death Donors Using Normothermic Regional Perfusion: Comparison With Liver Transplants From Brain Dead Donors. Transplant Proc 2019;51:12-9. [Crossref] [PubMed]
- Pearson R, Geddes C, Mark P, et al. Transplantation of kidneys after normothermic perfusion: A single center experience. Clin Transplant 2021;35:e14431. [Crossref] [PubMed]
- Ruiz P, Gastaca M, Bustamante FJ, et al. Favorable Outcomes After Liver Transplantation With Normothermic Regional Perfusion From Donors After Circulatory Death: A Single-center Experience. Transplantation 2019;103:938-43. [Crossref] [PubMed]
- Foss S, Nordheim E, Sørensen DW, et al. First Scandinavian Protocol for Controlled Donation After Circulatory Death Using Normothermic Regional Perfusion. Transplant Direct 2018;4:e366. [Crossref] [PubMed]
- Mowlem E, Randle L, Fear C, et al. Normothermic Regional Perfusion of Donors Following Circulatory Death Improves Outcomes in Liver Transplantation. Am J Transplant 2017;17:205-410.
- Miñambres E, Suberviola B, Dominguez-Gil B, et al. Improving the Outcomes of Organs Obtained From Controlled Donation After Circulatory Death Donors Using Abdominal Normothermic Regional Perfusion. Am J Transplant 2017;17:2165-72. [Crossref] [PubMed]
- Giadrosich BE, Riera MF, Canals LR. PD25-01 Kidney preservation techniques in controlled circulatory death: NECMO and ultra-rapid retrieval. J Urol 2018;199:e545-6.
- Oniscu GC, Randle LV, Muiesan P, et al. In situ normothermic regional perfusion for controlled donation after circulatory death--the United Kingdom experience. Am J Transplant 2014;14:2846-54. [Crossref] [PubMed]
- Rojas-Peña A, Sall LE, Gravel MT, et al. Donation after circulatory determination of death: the university of michigan experience with extracorporeal support. Transplantation 2014;98:328-34. [Crossref] [PubMed]
- Farney AC, Hines MH, al-Geizawi S, et al. Lessons learned from a single center's experience with 134 donation after cardiac death donor kidney transplants. J Am Coll Surg 2011;212:440-51; discussion 451-3. [Crossref] [PubMed]
- Lee CY, Tsai MK, Ko WJ, et al. Expanding the donor pool: use of renal transplants from non-heart-beating donors supported with extracorporeal membrane oxygenation. Clin Transplant 2005;19:383-90. [Crossref] [PubMed]
- Magliocca JF, Magee JC, Rowe SA, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma 2005;58:1095-101; discussion 1101-2. [Crossref] [PubMed]
- Gravel MT, Arenas JD, Chenault R 2nd, et al. Kidney transplantation from organ donors following cardiopulmonary death using extracorporeal membrane oxygenation support. Ann Transplant 2004;9:57-8. [PubMed]
- Antoine C, Savoye E, Gaudez F, et al. Kidney Transplant From Uncontrolled Donation After Circulatory Death: Contribution of Normothermic Regional Perfusion. Transplantation 2020;104:130-6. [Crossref] [PubMed]
- Molina M, Guerrero-Ramos F, Fernández-Ruiz M, et al. Kidney transplant from uncontrolled donation after circulatory death donors maintained by nECMO has long-term outcomes comparable to standard criteria donation after brain death. Am J Transplant 2019;19:434-47. [Crossref] [PubMed]
- Delsuc C, Faure A, Berthiller J, et al. Uncontrolled donation after circulatory death: comparison of two kidney preservation protocols on graft outcomes. BMC Nephrol 2018;19:3. [Crossref] [PubMed]
- Demiselle J, Augusto JF, Videcoq M, et al. Transplantation of kidneys from uncontrolled donation after circulatory determination of death: comparison with brain death donors with or without extended criteria and impact of normothermic regional perfusion. Transpl Int 2016;29:432-42. [Crossref] [PubMed]
- Reznik ON, Skvortsov AE, Reznik AO, et al. Uncontrolled donors with controlled reperfusion after sixty minutes of asystole: a novel reliable resource for kidney transplantation. PLoS One 2013;8:e64209. [Crossref] [PubMed]
- Valero R, Cabrer C, Oppenheimer F, et al. Normothermic recirculation reduces primary graft dysfunction of kidneys obtained from non-heart-beating donors. Transpl Int 2000;13:303-10. [Crossref] [PubMed]
- Fenner H, Buss C, Gardiner D. Intensive care staff attitudes to deceased organ donation. J Intensive Care Soc 2014;15:53-6. [Crossref]
- Croome KP, Barbas AS, Whitson B, et al. American Society of Transplant Surgeons recommendations on best practices in donation after circulatory death organ procurement. Am J Transplant 2023;23:171-9. [Crossref] [PubMed]
- Domínguez-Gil B, Ascher N, Capron AM, et al. Expanding controlled donation after the circulatory determination of death: statement from an international collaborative. Intensive Care Med 2021;47:265-81. [Crossref] [PubMed]
- Heide W. Non-heart-beating donors are ineligible. Nervenarzt 2016;87:161-8. [Crossref] [PubMed]
- UK Donation Ethics Committee. An Ethical framework for Controlled Donation after circulatory death. Academy of Royal Medical Colleges; 2011.
- Rand A, Koch T, Ragaller M. Organ donation-Not only a responsibility of intensive care medicine. Anaesthesist 2022;71:311-7. [Crossref] [PubMed]
- Bein T, Combes A, Meyfroidt G. Organ donation after controlled cardiocirculatory death: confidence by clarity. Intensive Care Med 2021;47:325-7. [Crossref] [PubMed]
- Lomero M, Gardiner D, Coll E, et al. Donation after circulatory death today: an updated overview of the European landscape. Transpl Int 2020;33:76-88. [Crossref] [PubMed]
- Shemie SD, Wilson LC, Hornby L, et al. A brain-based definition of death and criteria for its determination after arrest of circulation or neurologic function in Canada: a 2023 clinical practice guideline. Can J Anaesth 2023;70:483-557. [Crossref] [PubMed]
- Bernat JL, Domínguez-Gil B, Glazier AK, et al. Understanding the Brain-based Determination of Death When Organ Recovery Is Performed With DCDD In Situ Normothermic Regional Perfusion. Transplantation 2023;107:1650-4. [Crossref] [PubMed]
- Critical Care Canada Forum. The Paradigm shift to the Brain based definition and determination of Death. 2019. Available online: https://www.canadiancriticalcare.org/Webinar-Recordings
- Shemie SD, Hornby L, Baker A, et al. International guideline development for the determination of death. Intensive Care Med 2014;40:788-97. [Crossref] [PubMed]
- Dhanani S, Hornby L, van Beinum A, et al. Resumption of Cardiac Activity after Withdrawal of Life-Sustaining Measures. N Engl J Med 2021;384:345-52. [Crossref] [PubMed]
- Zorko DJ, Shemie J, Hornby L, et al. Autoresuscitation after circulatory arrest: an updated systematic review. Can J Anaesth 2023;70:699-712. [Crossref] [PubMed]
- Gordon AC, Hartle AJ. Donation after circulatory death--a new role for the anaesthetist? Anaesthesia 2011;66:761-4. [Crossref] [PubMed]
- NHS Novel Technologies Implementation Group. UK Protocol for Normothermic Regional Perfusion (NRP) in controlled Donation after Circulatory determination of Death; 2021.
- Schroder JN, Scheuer S, Catarino P, et al. The American Association for Thoracic Surgery 2023 Expert Consensus Document: Adult cardiac transplantation utilizing donors after circulatory death. J Thorac Cardiovasc Surg 2023;166:856-869.e5. [Crossref] [PubMed]
- Honarmand K, Alshamsi F, Foroutan F, et al. Antemortem Heparin in Organ Donation After Circulatory Death Determination: A Systematic Review of the Literature. Transplantation 2021;105:e337-46. [Crossref] [PubMed]
- Shahrestani S, Webster AC, Lam VW, et al. Outcomes From Pancreatic Transplantation in Donation After Cardiac Death: A Systematic Review and Meta-Analysis. Transplantation 2017;101:122-30. [Crossref] [PubMed]
- Roll GR, Quintini C, Reich DJ. In quest of the what, when, and where for machine perfusion dynamic liver preservation: Carpe diem! Liver Transpl 2022;28:1701-3. [Crossref] [PubMed]
- Mohkam K, Nasralla D, Mergental H, et al. In situ normothermic regional perfusion versus ex situ normothermic machine perfusion in liver transplantation from donation after circulatory death. Liver Transpl 2022;28:1716-25. [Crossref] [PubMed]
- Schroder JN, Patel CB, DeVore AD, et al. Transplantation Outcomes with Donor Hearts after Circulatory Death. N Engl J Med 2023;388:2121-31. [Crossref] [PubMed]
- Joshi Y, Villanueva J, Gao L, et al. Donation After Circulatory Death: A New Frontier. Curr Cardiol Rep 2022;24:1973-81. [Crossref] [PubMed]
- Alamouti-Fard E, Garg P, Wadiwala IJ, et al. Normothermic Regional Perfusion is an Emerging Cost-Effective Alternative in Donation After Circulatory Death (DCD) in Heart Transplantation. Cureus 2022;14:e26437. [Crossref] [PubMed]
- Gardiner D, Charlesworth M, Rubino A, et al. The rise of organ donation after circulatory death: a narrative review. Anaesthesia 2020;75:1215-22. [Crossref] [PubMed]
- Hessheimer AJ, Fondevila C. Normothermic Regional Perfusion in Solid Organ Transplantation. Advances in Extracorporeal Membrane Oxygenation 2019. doi:
10.5772/intechopen.84771 .10.5772/intechopen.84771 - Morrissey PE, Monaco AP. Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation 2014;97:258-64. [Crossref] [PubMed]
- Coll E, Miñambres E, Sánchez-Fructuoso A, et al. Uncontrolled Donation After Circulatory Death: A Unique Opportunity. Transplantation 2020;104:1542-52. [Crossref] [PubMed]
- NHS Novel Technologies Implementation Group. UK National Protocol for direct retrieval and perfusion (DRP) of DCD Hearts and Lungs with or without abdominal NRP (A-NRP) to Ex-situ Normothermic perfusion; 2021.
- Lazzeri C, Bonizzoli M, Valente S, et al. The role of extracorporeal membrane oxygenation in donation after circulatory death. Minerva Anestesiol 2014;80:1217-27. [PubMed]
- Jochmans I, Hessheimer AJ, Neyrinck AP, et al. Consensus statement on normothermic regional perfusion in donation after circulatory death: Report from the European Society for Organ Transplantation's Transplant Learning Journey. Transpl Int 2021;34:2019-30. [Crossref] [PubMed]
- European Society of Organ Transplantation. Establishing a Normothermic Regional Perfusion Programme. ESOT Congress 2021: Pre-Congress Webinar Series 2021. Available online: https://www.youtube.com/watch?v=Yx5W-U2dtHo
- Hessheimer AJ, Polak W, Antoine C, et al. Regulations and Procurement Surgery in DCD Liver Transplantation: Expert Consensus Guidance From the International Liver Transplantation Society. Transplantation 2021;105:945-51. [Crossref] [PubMed]
- Naranjo Gozalo S, Ballesteros Sanz MLA, Alvarez De Arriba C, et al. Lung Rapid Recovery Procurement Combined with Abdominal Normothermic Regional Perfusion in Controlled Donation after Circulatory Death. J Vis Exp 2022; [Crossref] [PubMed]
- Malas J, Chen Q, Thomas J, et al. The impact of thoracoabdominal normothermic regional perfusion on early outcomes in donation after circulatory death lung transplantation. J Heart Lung Transplant 2023;42:1040-4. [Crossref] [PubMed]
- Large S, Messer S, Berman M, et al. Protocol: Thoraco-Abdominal Normothermic Regional Perfusion (TANRP). National TANRP protocol for DCD organ retrieval; 2018.
- Hunt F, Johnston CJC, Coutts L, et al. From Haphazard to a Sustainable Normothermic Regional Perfusion Service: A Blueprint for the Introduction of Novel Perfusion Technologies. Transpl Int 2022;35:10493. [Crossref] [PubMed]
- Giske L, Solberg B, Tranvåg E, et al. Organ donation with the use of normothermic regional perfusion in patients who die after cardiac and respiratory arrest after withdrawal of life-sustaining treatment. Norwegian Institute of Public Health; 2019.
- Murphy N, Weijer C, Smith M, et al. Controlled Donation After Circulatory Determination of Death: A Scoping Review of Ethical Issues, Key Concepts, and Arguments. J Law Med Ethics 2021;49:418-40. [Crossref] [PubMed]
- Wind J. Critical issues in donation after circulatory death. [Doctoral Thesis, Maastricht University]. Datawyse/Universitaire Pers Maastricht. 2016. doi:
10.26481/dis.20160219jw .10.26481/dis.20160219jw - Price D, Danbury C. Best Interests is Best Practice: Law and Donation Masterclass. Professional Development Programme for Organ Donation: NHS Blood and Transfusion; 2010. Available online: https://www.slideserve.com/akiko/law-and-donation-masterclass-best-interests-is-best-practice
- Manara A, Shemie SD, Large S, et al. Maintaining the permanence principle for death during in situ normothermic regional perfusion for donation after circulatory death organ recovery: A United Kingdom and Canadian proposal. Am J Transplant 2020;20:2017-25. [Crossref] [PubMed]
- American College of Physicians. Ethics, determination of death, and organ transplantation in normothermic regional perfusion (NRP) with controlled donation after circulatory determination of death (cDCD): American College of Physicians Statement of Concern; 2021.
- Wall AE, Fiedler A, Karp S, et al. Applying the ethical framework for donation after circulatory death to thoracic normothermic regional perfusion procedures. Am J Transplant 2022;22:1311-5. [Crossref] [PubMed]
- Parent B, Caplan A, Moazami N, et al. Response to American College of Physician's statement on the ethics of transplant after normothermic regional perfusion. Am J Transplant 2022;22:1307-10. [Crossref] [PubMed]
- Lazaridis C. Normothermic regional perfusion: Ethically not merely permissible but recommended. Am J Transplant 2022;22:2285-6. [Crossref] [PubMed]
- Entwistle JW, Drake DH, Fenton KN, et al. Normothermic Regional Perfusion: Ethical Issues in Thoracic Organ Donation. Ann Thorac Surg 2022;114:44-51. [Crossref] [PubMed]
- Peled H, Mathews S, Rhodes D, et al. Normothermic Regional Perfusion Requires Careful Ethical Analysis Before Adoption Into Donation After Circulatory Determination of Death. Crit Care Med 2022;50:1644-8. [Crossref] [PubMed]
- Glazier AK, Capron AM. Normothermic regional perfusion and US legal standards for determining death are not aligned. Am J Transplant 2022;22:1289-90. [Crossref] [PubMed]
- Murphy NB, Weijer C, Slessarev M, et al. Implications of the updated Canadian Death Determination Guidelines for organ donation interventions that restore circulation after determination of death by circulatory criteria. Can J Anaesth 2023;70:591-5. [Crossref] [PubMed]
Cite this article as: O’Rourke J, Crowe G, Turner R, Gaffney A. Donation after circulatory death: a narrative review of current controversies, attitudes, and the evolving role of regional perfusion technology. AME Med J 2024;9:9.