
Early versus late intervention for degenerative cervical myelopathy: what are the outcomes?—a review of the current literature
What is degenerative cervical myelopathy (DCM)?
DCM, or cervical spondylotic myelopathy, is a condition resulting from chronic compression of the spinal cord in the cervical spine. Patient presentation may vary, though symptoms typically include gait disturbance, balance and coordination issues, hyperreflexia, and/or loss of finger dexterity. Additionally, DCM can manifest with autonomic symptoms such as bowel or bladder dysfunction. These patients commonly have neck pain, stiffness, and may have concomitant cervical radiculopathy affecting one or more nerve distributions (1).
Degeneration in the cervical spine is quite common in patients older than 50 years old. However, only a small portion of these patients will exhibit symptoms of DCM (2,3). A number of studies have shown that a disproportionate number of patients with DCM have some form of preexisting spinal canal narrowing or congenital stenosis of the cervical spine which may predispose them to developing DCM (4-7). These studies typically defined congenital stenosis as a canal sagittal diameter of <13 mm.
DCM is generally considered a progressive disease, with patients exhibiting a stepwise decline in function (7). However, the rate at which DCM progresses can vary widely from patient to patient. This variability in clinical course can make both the diagnosis and management of DCM challenging for providers (8). Furthermore, the exact impact of surgical intervention timing on the natural history of the DCM is an area of ongoing research (9).
Pathophysiology of DCM
The pathophysiology of DCM is related to both heritable and environmental contributions (6). Degenerative changes in the cervical spine occurring as a result of aging and repetitive stress cause structural changes in both the bone and soft tissues surrounding the spinal cord. Anteriorly, disc degeneration and herniation, hypertrophy or ossification of the posterior longitudinal ligament, and uncovertebral osteophyte formation can contribute to stenosis. Posteriorly, hypertrophy or infolding of the ligamentum flavum, as well as degeneration of the facet joints, further reduces space available for the spinal cord. Dynamic compression from pathologic movement or instability of the cervical spine may compound these static changes. Figure 1 demonstrates the most common etiologies of spinal cord compression in DCM.
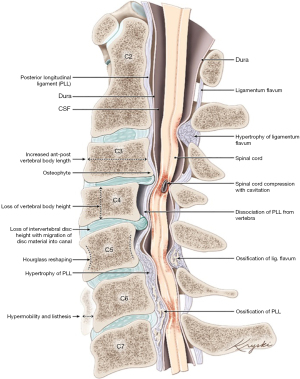
Sources of mechanical compression on the cervical spine create microvascular compromise in the spinal cord resulting in ischemia and inflammation, ultimately leading to demyelination, axonal degeneration, and neuronal degradation (1). Ischemia is supported by the literature as a major underlying pathologic process in DCM (11-13). One cell type that is particularly sensitive to ischemic injury is the oligodendrocyte which is primarily responsible for insulating axons with myelin sheaths (14). Based on human and animal studies, oligodendroglial apoptotic death related to ischemia is seen in the same location as demyelinated axons (15,16). Based on oligodendrocytes known role in insulating axons and maintaining axonal integrity, it follows that axonal demyelination and ultimate destruction in the setting of ischemia may be preceded by apoptosis of the surrounding oligodendrocytes (15,16). The progressive apoptotic loss of neuroglial cells and subsequent axonal degeneration is expressed as the progressive neurological deficits seen clinically with DCM (17).
Epidemiology
The true incidence of DCM is not well-defined given its relative diagnostic complexity, but is the most common cause of spinal cord dysfunction in those greater than 50 years old (8,18,19). The prevalence of operatively-treated DCM is estimated to be 1.6 per 100,000, though this number likely greatly underestimates the actual prevalence of DCM in the general population (20). DCM is more prevalent in males than females, and patients are most commonly initially diagnosed in their 50s (18). DCM is an increasingly important clinical consideration given that its incidence is projected to increase dramatically with the aging population (21,22).
How is DCM diagnosed?
Clinical evaluation
DCM is a clinical diagnosis that requires careful correlation between patients’ history, physical examination, and imaging. The presenting symptoms of DCM are often quite subtle and there is considerable variability in symptoms between patients. Additionally, DCM’s symptoms often overlap with other neurological conditions leading to potential misdiagnosis. Specifically, one previous report showed that a significant portion of patients with DCM associated hand numbness were initially misdiagnosed in the community with carpal tunnel syndrome (23). Other disorders with which DCM can easily be confused are multiple sclerosis, intracranial pathology, normal pressure hydrocephalus, vitamin B12 deficiency, and amyotrophic lateral sclerosis (24). To make matters more difficult, there are no universal criteria for diagnosis of DCM (25,26).
In the upper extremity, patients often present with loss of dexterity and coordination in the hands. In the lower extremities, patients commonly experience gait and/or balance disturbances (27). Additionally, DCM can manifest with autonomic symptoms such as bowel or bladder incontinence, retention, or erectile dysfunction (27). Clinicians must be able to distinguish between cervical radicular pain and myelopathic features, which can often coexist (27). Furthermore, some patients may present with atypical symptoms of DCM including blurred vision, headache, nausea, palpitation, tinnitus, vertigo, hypomnesia, and abdominal discomfort (28,29).
There are multiple physical exam findings described as associated with DCM. A systematic review evaluated each of these physical exam tests based on their diagnostic accuracy and found that the most sensitive tests were the inverted brachioradialis sign (61%), hyperreflexic patellar tendon reflex (56%), and Hoffmann’s sign (44%). The most specific signs were sustained clonus (92%) and Babinski sign (96%) (30). One recent study found a diagnostic specificity of 94–99% in patients who exhibited 3 of 5 of the following clinical indicators: gait deviation, positive Hoffmann’s test, inverted supinator sign, positive Babinski test, and age >45 years. In those with ≤1 of these symptoms, the likelihood of DCM is low with a negative likelihood ratio of 0.18 (31). Importantly, many patients do not present with all or any of these physical exam findings, with up to 20% of myelopathic patients not exhibiting any of these discrete exam findings (32).
Several patient reported outcomes have been created to grade and monitor the severity and progression of DCM. The most commonly used outcomes are the Japanese Orthopaedic Association (JOA) and modified JOA (mJOA) scoring systems (33). This classification system classifies patients as mild (mJOA 15–17), moderate (mJOA 12–14), or severe (mJOA 0–11). In brief, more severe upper and lower extremity sensory and motor deficits and bladder dysfunction lead to more severe disease classification. The inter-reader variability of the mJOA score is reported as good in the literature, though it is important to remember that these measurements are imperfect and standardizing the clinical assessment of DCM remains difficult (34).
Another major classification system for DCM is the Nurick grading system, which aims to correlate degree of cord compression to symptom severity (35,36). Nurick grades span from 0 to 6, with 0 indicating clinical evidence of root involvement but no evidence of spinal cord compression, and 5 indicating that the patient is bedridden. The Nurick scale is more lower extremity focused and has been found less sensitive than mJOA (37). One recent study endorses the use of the National Institutes of Health (NIH) Toolbox as a sensitive and quantitative evaluation tool for DCM (38,39). The NIH Toolbox includes objective measure of motor, sensory, cognitive, and emotional dysfunction including physical tests instead of questions on a questionnaire. Finally, there are numerous additional commonly used measures including but not limited to the Prolo Scale and Neck Disability Index, the details of which may be outside the scope of the current article (40,41).
Imaging evaluation
Magnetic resonance imaging (MRI) is the gold standard imaging modality as part of the diagnosis of DCM. MRI directly visualizes the degree of stenosis and cord compression and can display intramedullary spinal cord signal change (42). Examples of MRI findings in mild and severe DCM are provided in Figures 2,3, respectively. There are many advanced MRI techniques which can be employed to evaluate the specific microstructural features of DCM including diffusion tensor imaging (DTI), magnetization transfer (MT), myelin water fraction (MWF), and magnetic resonance spectroscopy (MRS) (43,44). Additionally, functional MRI may add to our understanding of the upstream functional effects of DCM in the brain (45). With the exception of DTI, data supporting widespread routine use of these metrics is somewhat limited to date. However, they may be a promising step towards earlier diagnosis of DCM pending further study (44,46). DTI has been shown to reliably distinguish between Nurick grades in a prospective study (47). Asymptomatic cord compression on imaging is prevalent in the general population (48-50), and it is important to remember that both MRI evidence of central stenosis and the presence of neurologic symptoms consistent with myelopathy must be present in order to make a diagnosis of DCM. Patients with imaging findings of central stenosis without clinical symptoms should be educated to remain vigilant for the development of myelopathic symptoms.
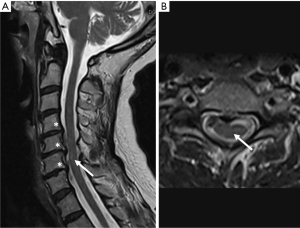
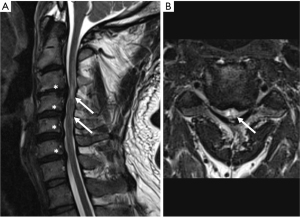
How is DCM treated?
The decision between nonoperative and operative management is typically based on the severity of disease and evidence of disease progression. In cases of moderate to severe DCM, surgical intervention is recommended. In cases of mild DCM, it is reasonable to offer patients a choice between surgical intervention and a trial of rehabilitation under close surveillance. If there is evidence of neurologic deterioration during a trial of conservative treatment, the patient should undergo decompressive surgery (51). Incidental imaging findings of cord compression in the absence of clinical symptoms of DCM are not an indication for surgery (22). Surgical management of DCM involves decompression of the involved spinal levels which can be achieved by several different surgical techniques from either an anterior or posterior approach to the cervical spine. Both anterior and posterior approaches to cervical decompression have been validated as efficacious, but the specific technique and approach used may depend on patient factors, surgeon preference, number of levels involved and sagittal alignment of the cervical spine (52).
How does surgical timing affect outcomes?
Several high-quality studies have shown that surgical decompression is an effective treatment method for DCM (53-56). Specifically, the goal of surgical treatment is to halt disease progression. Typically decompressive surgery also leads to improved function, pain, and quality of life (53-56). However, the effect of surgical timing on surgical efficacy is less clear. Previous literature has defined timing of operative intervention in two primary ways: (I) duration of patients’ symptoms and (II) disease severity based on JOA, mJOA, or other DCM scores.
When examining outcomes based on duration of symptoms, the literature is inconclusive. Several studies have concluded that all patients can expect to see symptomatic and neurological improvements based on postoperative JOA and mJOA scores irrespective of symptom duration (57-60). These articles are contradicted by other large multicenter studies and national registry data which have concluded that, although patients can expect to benefit from surgery, those who have more longstanding symptoms may experience a greater degree of residual disability (61-63). The odds of achieving an mJOA score >16 decreased by 22% in a stepwise fashion from shorter to longer symptom duration, with durations of symptoms stratified as <3, 3–6, 7–12, 13–24, and >24 months (62).
When defining intervention timing by disease severity scores, the literature is more unanimous that patients with greater severity of disease have a lower chance of returning to normal functional and neurological status (53,62,64,65). Patients with severe myelopathy (<12 mJOA scores) achieved the greatest improvement in score from baseline after surgery, but still achieved a postoperative mJOA score that was lower than those who started with mJOA scores ≥15 (53). The chances of achieving an mJOA >16 at 1 year postoperatively increased by 22% for every one point increase in preoperative mJOA score, indicating that those with more mild disease had superior absolute outcomes (62). Another study specifically found that patients with very severe (mJOA <8) or severe DCM (mJOA 9–11) improved from preoperative status, but had significant residual disability (65).
The heterogeneity in conclusions based on how timing of surgery is defined may, in part, be due to the significant variability in the timeline of disease progression. Indeed, several high quality studies have found no correlation between a patient’s disease severity based on JOA or mJOA scores and the duration of their symptoms (61,66). The authors therefore feel that disease severity based on JOA or mJOA score may be a more reproducible and less confounded way to quantify surgical timing given the variability in disease progression between patients. Additionally, the degree of spinal cord injury on MRI may also lend insight into disease severity and can impact surgical decision making. Several studies have found high intensity signal within the spinal cord on MRI to be a predictor of worse neurologic outcomes after surgical intervention (66-68).
There does remain a subset of patients who have some form of transient neurologic decline post decompression, and one mechanism posited to be responsible is ischemia-reperfusion injury (IRI) (69). One animal study performed by Vidal et al. investigated the effects of early versus delayed intervention on DCM on neurological outcomes and examined the inflammatory response to decompression in each study group, which is thought to be the underlying mechanism for IRI (70). Interestingly, they found that the rats with delayed intervention had a more prolonged period of increased cytokine response, astroglial apoptosis, and inflammatory monocytes and this correlated with lack of neurological improvement when compared to the early intervention group (70).
Overall, the literature suggests that early surgical intervention is essential to minimizing long-term disability and maximizing quality of life. Regardless of the metric used for surgical timing, patients with symptomatic and worsening DCM benefit from surgical decompression and can expect a halt in disease progression and at least some meaningful functional improvement.
Limitations
The present article is not intended as a systematic review or meta-analysis of all studies to date, but rather to present a concise and meaningful guide for identifying, diagnosing, and managing DCM, with a special focus on the importance of intervention timing. The article is aimed at a full range of readers, from general practitioners to the practicing spine surgeon. No novel data is presented in the current article.
Conclusions
- DCM is an important consideration in patients over 50 with progressive neurological symptoms including, but not limited to, gait and/or balance disturbances, loss of hand dexterity, and neck stiffness and pain.
- Patients with suspected DCM based on history, physical exam, and/or MRI findings should be referred to a spine specialist promptly.
- Early surgical intervention is essential to limit long-term functional disability and maximize quality of life in patients with DCM.
- Patients with symptomatic and worsening DCM benefit from surgical decompression and can expect a halt in disease progression and at least some meaningful improvement in neurologic function.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Degenerative Spine Disease”. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-196/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-196/coif). The series “Degenerative Spine Disease” was commissioned by the editorial office without any funding or sponsorship. B.A.K. served as the unpaid Guest Editor of the series. W.R.S. reports medical education work for Stryker and leadership on several Spine Surgery Societies (NASS, CSRS, AO North America). B.L. reports consulting fees from Medtronic; participation in the Medtronic biologics advisory board for spine related fusion technologies. D.S.B. reports royalties from CTL Amedica, consulting for Orthofix posterior cervical implant product development, and participation as keynote speaker at the Stryker Fellows Meeting. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gharooni AA, Kwon BK, Fehlings MG, et al. Developing Novel Therapies for Degenerative Cervical Myelopathy [AO Spine RECODE-DCM Research Priority Number 7]: Opportunities From Restorative Neurobiology. Global Spine J 2022;12:109S-121S. [Crossref] [PubMed]
- Irvine DH, Foster JB, Newell DJ, et al. Prevalence of cervical spondylosis in a general practice. Lancet 1965;1:1089-92. [Crossref] [PubMed]
- Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondylotic cervical cord compression. Spine (Phila Pa 1976) 2004;29:2260-9. [Crossref] [PubMed]
- Wilson JR, Barry S, Fischer DJ, et al. Frequency, timing, and predictors of neurological dysfunction in the nonmyelopathic patient with cervical spinal cord compression, canal stenosis, and/or ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2013;38:S37-54. [Crossref] [PubMed]
- Edwards WC, LaRocca SH. The developmental segmental sagittal diameter in combined cervical and lumbar spondylosis. Spine (Phila Pa 1976) 1985;10:42-9. [Crossref] [PubMed]
- Patel AA, Spiker WR, Daubs M, et al. Evidence of an inherited predisposition for cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2012;37:26-9. [Crossref] [PubMed]
- Morishita Y, Naito M, Hymanson H, et al. The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J 2009;18:877-83. [Crossref] [PubMed]
- Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 2013;19:409-21. [Crossref] [PubMed]
- Ganau M, Holly LT, Mizuno J, et al. Future Directions and New Technologies for the Management of Degenerative Cervical Myelopathy. Neurosurg Clin N Am 2018;29:185-93. [Crossref] [PubMed]
- Gibson J, Nouri A, Krueger B, et al. Degenerative Cervical Myelopathy: A Clinical Review. Yale J Biol Med 2018;91:43-8. [PubMed]
- Brain WR, Knight GC, Bull JW. Discussion of rupture of the intervertebral disc in the cervical region. Proc R Soc Med 1948;41:509-16. [Crossref] [PubMed]
- Gooding MR, Wilson CB, Hoff JT. Experimental cervical myelopathy. Effects of ischemia and compression of the canine cervical spinal cord. J Neurosurg 1975;43:9-17. [Crossref] [PubMed]
- Gooding MR, Wilson CB, Hoff JT. Experimental cervical myelopathy: autoradiographic studies of spinal cord blood flow patterns. Surg Neurol 1976;5:233-9. [PubMed]
- Crowe MJ, Bresnahan JC, Shuman SL, et al. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 1997;3:73-6. [Crossref] [PubMed]
- Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma 1985;2:299-315. [Crossref] [PubMed]
- Bunge RP, Puckett WR, Becerra JL, et al. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 1993;59:75-89. [PubMed]
- Kim DH, Vaccaro AR, Henderson FC, et al. Molecular biology of cervical myelopathy and spinal cord injury: role of oligodendrocyte apoptosis. Spine J 2003;3:510-9. [Crossref] [PubMed]
- Northover JR, Wild JB, Braybrooke J, et al. The epidemiology of cervical spondylotic myelopathy. Skeletal Radiol 2012;41:1543-6. [Crossref] [PubMed]
- Simeone FA, Rothman RN. Cervical Disc Disease. 2nd ed. Philadelphia, PA: Saunders; 1982.
- Boogaarts HD, Bartels RH. Prevalence of cervical spondylotic myelopathy. Eur Spine J 2015;24:139-41. [Crossref] [PubMed]
- Nouri A, Tetreault L, Singh A, et al. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976) 2015;40:E675-93. [Crossref] [PubMed]
- Kovalova I, Kerkovsky M, Kadanka Z, et al. Prevalence and Imaging Characteristics of Nonmyelopathic and Myelopathic Spondylotic Cervical Cord Compression. Spine (Phila Pa 1976) 2016;41:1908-16. [Crossref] [PubMed]
- Behrbalk E, Salame K, Regev GJ, et al. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg Focus 2013;35:E1. [Crossref] [PubMed]
- Milligan J, Ryan K, Fehlings M, et al. Degenerative cervical myelopathy: Diagnosis and management in primary care. Can Fam Physician 2019;65:619-24. [PubMed]
- Davies BM, McHugh M, Elgheriani A, et al. The reporting of study and population characteristics in degenerative cervical myelopathy: A systematic review. PLoS One 2017;12:e0172564. [Crossref] [PubMed]
- Davies BM, Munro CF, Kotter MR. A Novel Insight Into the Challenges of Diagnosing Degenerative Cervical Myelopathy Using Web-Based Symptom Checkers. J Med Internet Res 2019;21:e10868. [Crossref] [PubMed]
- Harrop JS, Hanna A, Silva MT, et al. Neurological manifestations of cervical spondylosis: an overview of signs, symptoms, and pathophysiology. Neurosurgery 2007;60:S14-20. [Crossref] [PubMed]
- Sun Y, Muheremu A, Tian W. Atypical symptoms in patients with cervical spondylosis: Comparison of the treatment effect of different surgical approaches. Medicine (Baltimore) 2018;97:e10731. [Crossref] [PubMed]
- Serre H, Labauge R, Simon L, et al. Barré-Liéou syndrome, designated "posterior sympathetic cervical syndrome". Rhumatologie 1969;21:217-46. [PubMed]
- Cook CE, Wilhelm M, Cook AE, et al. Clinical tests for screening and diagnosis of cervical spine myelopathy: a systematic review. J Manipulative Physiol Ther 2011;34:539-46. [Crossref] [PubMed]
- Cook C, Brown C, Isaacs R, et al. Clustered clinical findings for diagnosis of cervical spine myelopathy. J Man Manip Ther 2010;18:175-80. [Crossref] [PubMed]
- Rhee JM, Heflin JA, Hamasaki T, et al. Prevalence of physical signs in cervical myelopathy: a prospective, controlled study. Spine (Phila Pa 1976) 2009;34:890-5. [Crossref] [PubMed]
- Tetreault L, Kopjar B, Nouri A, et al. The modified Japanese Orthopaedic Association scale: establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J 2017;26:78-84. [Crossref] [PubMed]
- Martin AR, Jentzsch T, Wilson JRF, et al. Inter-rater Reliability of the Modified Japanese Orthopedic Association Score in Degenerative Cervical Myelopathy: A Cross-sectional Study. Spine (Phila Pa 1976) 2021;46:1063-9. [Crossref] [PubMed]
- Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain 1972;95:87-100. [Crossref] [PubMed]
- Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain 1972;95:101-8. [Crossref] [PubMed]
- Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J 2015;24:236-51. [Crossref] [PubMed]
- Hodes RJ, Insel TR, Landis SC, et al. The NIH toolbox: setting a standard for biomedical research. Neurology 2013;80:S1. [Crossref] [PubMed]
- Muhammad F, Baha A, Haynes G, et al. Isolating Neurologic Deficits in Cervical Spondylotic Myelopathy: A Case-Controlled Study, Using the NIH Toolbox Motor Battery. Neurol Clin Pract 2023;13:e200126. [Crossref] [PubMed]
- Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusions. Spine (Phila Pa 1976) 1986;11:601-6. [Crossref] [PubMed]
- Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991;14:409-15. [PubMed]
- Nagata K, Kiyonaga K, Ohashi T, et al. Clinical value of magnetic resonance imaging for cervical myelopathy. Spine (Phila Pa 1976) 1990;15:1088-96. [Crossref] [PubMed]
- Martin AR, Aleksanderek I, Cohen-Adad J, et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuroimage Clin 2016;10:192-238. [Crossref] [PubMed]
- Badhiwala JH, Ahuja CS, Akbar MA, et al. Degenerative cervical myelopathy - update and future directions. Nat Rev Neurol 2020;16:108-24. [Crossref] [PubMed]
- Khan AF, Muhammad F, Mohammadi E, et al. Beyond the aging spine - a systematic review of functional changes in the human brain in cervical spondylotic myelopathy. Geroscience 2024;46:1421-50. [Crossref] [PubMed]
- Martin AR, De Leener B, Cohen-Adad J, et al. Can microstructural MRI detect subclinical tissue injury in subjects with asymptomatic cervical spinal cord compression? A prospective cohort study. BMJ Open 2018;8:e019809. [Crossref] [PubMed]
- Rajasekaran S, Yerramshetty JS, Chittode VS, et al. The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine (Phila Pa 1976) 2014;39:1183-9. [Crossref] [PubMed]
- Teresi LM, Lufkin RB, Reicher MA, et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology 1987;164:83-8. [Crossref] [PubMed]
- Boden SD, McCowin PR, Davis DO, et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990;72:1178-84. [Crossref] [PubMed]
- Kato F, Yukawa Y, Suda K, et al. Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: Magnetic resonance imaging of over 1,200 asymptomatic subjects. Eur Spine J 2012;21:1499-507. [Crossref] [PubMed]
- Fehlings MG, Tetreault LA, Riew KD, et al. A Clinical Practice Guideline for the Management of Degenerative Cervical Myelopathy: Introduction, Rationale, and Scope. Global Spine J 2017;7:21S-7S. [Crossref] [PubMed]
- Ghogawala Z, Terrin N, Dunbar MR, et al. Effect of Ventral vs Dorsal Spinal Surgery on Patient-Reported Physical Functioning in Patients With Cervical Spondylotic Myelopathy: A Randomized Clinical Trial. JAMA 2021;325:942-951. [Crossref] [PubMed]
- Fehlings MG, Wilson JR, Kopjar B, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am 2013;95:1651-8. [Crossref] [PubMed]
- Johansen TO, Vangen-Lønne V, Holmberg ST, et al. Surgery for degenerative cervical myelopathy in the elderly: a nationwide registry-based observational study with patient-reported outcomes. Acta Neurochir (Wien) 2022;164:2317-26. [Crossref] [PubMed]
- Khan I, Archer KR, Wanner JP, et al. Trajectory of Improvement in Myelopathic Symptoms From 3 to 12 Months Following Surgery for Degenerative Cervical Myelopathy. Neurosurgery 2020;86:763-8. [Crossref] [PubMed]
- Fehlings MG, Ibrahim A, Tetreault L, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine (Phila Pa 1976) 2015;40:1322-8. [Crossref] [PubMed]
- Baumann AN, Chen M, Ahorukomeye P, et al. Factors Associated With the Rate of Recovery After Cervical Decompression Surgery for Degenerative Cervical Myelopathy: A Retrospective Analysis. Cureus 2023;15:e39654. [Crossref] [PubMed]
- Suzuki A, Misawa H, Simogata M, et al. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976) 2009;34:2874-9. [Crossref] [PubMed]
- Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013;38:392-400. [Crossref] [PubMed]
- Asuzu DT, Yun JJ, Alvi MA, et al. Association of ≥12 months of delayed surgical treatment for cervical myelopathy with worsened postoperative outcomes: a multicenter analysis of the Quality Outcomes Database. J Neurosurg Spine 2022;36:568-74. [Crossref] [PubMed]
- Tetreault L, Wilson JR, Kotter MRN, et al. Is Preoperative Duration of Symptoms a Significant Predictor of Functional Outcomes in Patients Undergoing Surgery for the Treatment of Degenerative Cervical Myelopathy? Neurosurgery 2019;85:642-7. [Crossref] [PubMed]
- Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am 2013;95:1659-66. [Crossref] [PubMed]
- Gerdhem L, Charalampidis A, Gerdhem P. Patient-reported Data as Predictors of Surgical Outcome in Patients With Degenerative Cervical Myelopathy: Analysis of a National Multicenter Dataset. Spine (Phila Pa 1976) 2023;48:113-9. [Crossref] [PubMed]
- Toci GR, Canseco JA, Karamian BA, et al. The impact of preoperative neurological symptom severity on postoperative outcomes in cervical spondylotic myelopathy. J Craniovertebr Junction Spine 2022;13:94-100. [Crossref] [PubMed]
- Kopjar B, Bohm PE, Arnold JH, et al. Outcomes of Surgical Decompression in Patients With Very Severe Degenerative Cervical Myelopathy. Spine (Phila Pa 1976) 2018;43:1102-9. [Crossref] [PubMed]
- Gembruch O, Jabbarli R, Rashidi A, et al. Surgery for Degenerative Cervical Myelopathy: What Really Counts? Spine (Phila Pa 1976) 2021;46:294-9. [Crossref] [PubMed]
- Suri A, Chabbra RP, Mehta VS, et al. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J 2003;3:33-45. [Crossref] [PubMed]
- Wada E, Yonenobu K, Suzuki S, et al. Can intramedullary signal change on magnetic resonance imaging predict surgical outcome in cervical spondylotic myelopathy? Spine (Phila Pa 1976) 1999;24:455-61; discussion 462. [Crossref] [PubMed]
- Karadimas SK, Laliberte AM, Tetreault L, et al. Riluzole blocks perioperative ischemia-reperfusion injury and enhances postdecompression outcomes in cervical spondylotic myelopathy. Sci Transl Med 2015;7:316ra194. [Crossref] [PubMed]
- Vidal PM, Karadimas SK, Ulndreaj A, et al. Delayed decompression exacerbates ischemia-reperfusion injury in cervical compressive myelopathy. JCI Insight 2017;2:e92512. [Crossref] [PubMed]
Cite this article as: Connelly JW, Kothari P, Spina N, Spiker WR, Lawrence B, Brodke DS, Karamian BA. Early versus late intervention for degenerative cervical myelopathy: what are the outcomes?—a review of the current literature. AME Med J 2024;9:14.

