Evaluating the feasibility of exosome technologies in COVID-19 treatment: navigating the intersection of reality and fantasy perspectives
Introduction
Since its first identification in Wuhan, China in December 2019, the coronavirus disease 2019 (COVID-19) disease caused by the novel coronavirus has been classified as a global public health emergency. Six months following the initial diagnosis of COVID-19, there was a notable absence of effective antiviral medication or vaccine to address this pressing public health crisis. Upon contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the patient’s primary defence mechanism is their immune system, with supplementary medical intervention administered in the event of any ensuing complications . The preliminary phase of SARS-CoV-2 pathogenesis has been confirmed to entail the specific recognition of the angiotensin-converting enzyme 2 receptor (ACE2) by the viral protein spike (1). The overactive body’s defence observed in COVID-19 patients has been linked with the substantial production of inflammatory mediators, which stimulate a cytokine storm marked by an overabundance of natural killer cells and T cells with effector properties (2,3). The frequency of admission rates to the intensive care unit (ICU) following a confirmed COVID-19 diagnosis is slightly above 5% (4). A significant proportion of ICU patients showed elevated levels of granulocyte colony-stimulating factor (GCSF) and tumor necrosis factor-alpha (TNF-α) in their plasma, with over 50% displaying these markers (5). The elevated GCSF and TNF-α levels can trigger a cytokine storm, leading to acute respiratory distress syndrome (ARDS) (1). The COVID-19-affected patient exhibited elevated levels of pro-inflammatory cytokines, specifically GCSF, Interferon-gamma inducible-10, and TNF-α. The concentrations of these cytokines were notably higher in patients who required admission to the ICU (6). The cytokine storm is responsible for causing severe damage to the lungs, which can ultimately result in fatality. Hence, the potential effectiveness of a treatment strategy for COVID-19 lies in the eradication of the cytokine storm and in depleting the response of super-inflammatory, coupled with the restoration and regeneration of pulmonary tissues (7).
The current therapeutic modalities and clinical interventions utilized for acute lung injuries linked to COVID-19 encompass respiratory support techniques, including ventilation support, antipyretic, non-specific antiviral, corticosteroids, and immunomodulatory therapy (1). Except remdesivir, which functions as an anti-Ebola virus medication, along with favipiravir, no effective therapeutic drug has been identified for the management of COVID-19 (8). The pandemic has contributed to the development and implementation of novel and effective COVID-19 treatments. By employing the body’s innate immune mechanism for regenerating and repairing damaged or diseased organs and tissues, cell-based therapies have been devised for managing the condition (9). The area in question has experienced significant growth in recent times to respond to the requirements of patients with diverse medical conditions, ranging from simple to complex (7).
Cell-based therapies target slowing down or preventing destructive or pathophysiological processes that eventually lead to symptomatic diseases (7). Numerous regenerative therapies have been discovered to activate the innate repair mechanism by regulating the functions of somatic and progenitor cells, thus preventing degeneration and stimulating regeneration (7,10). The majority of eukaryotic cells can produce and release extracellular vesicles (EVs) (11). These diminutive vesicles play a substantial role in injury, inflammation, and infections, and are categorized into distinct subtypes such as exosomes, and apoptotic bodies (ABs) (12). These groups exhibit notable distinctions in their respective contents, biogenesis pathways, and functional roles (12).
Recent research has emphasized the notable involvement of EVs and exosomes in the context of viral infections. This focus stems from the release of EVs harboring viral components, which have the potential to initiate viral infection in adjacent healthy cells (13). Exosomes, derived from diverse cellular sources including immune cells, encapsulate a diverse array of bioactive constituents like proteins, lipids, and nucleic acids (14). The transmission electron microscopy image displaying exosomes derived from the culture media of an oral squamous cell carcinoma cell line signifies the diversity of exosome origins from various cell sources. This variability significantly influences their functions in intercellular communication and therapeutic applications. as depicted in Figure 1. Exosomes have anti-inflammatory, immunomodulatory, and regenerative capabilities, making them a prospective COVID-19 treatment candidate (15). Recent studies have demonstrated the potential therapeutic effects of exosomes derived from mesenchymal stem cells (MSCs) in relation to COVID-19 (16,17). These MSCs are recognized for their immune-regulatory and anti-inflammatory characteristics, attributes that extend to the exosomes they release, which exhibit similar properties (17). Experimental evidence has demonstrated that exosomes produced from MSCs play a significant role in reducing the severity of lung injury and improving pulmonary function in animal models of COVID-19. Moreover, it has been observed that exosomes produced from MSCs have the capability to impede the reproduction of SARS-CoV-2 in controlled in vitro settings (18). Additional research has highlighted the potential of exosomes derived from immune cell subsets like dendritic cells and macrophages for potential therapeutic applications within the framework of COVID-19. These exosomes possess the ability to elicit an immune reaction targeted at the virus, a phenomenon that has been demonstrated in vitro antiviral properties (19). A notable challenge in exosome therapy is developing effective and safe ways to isolate, purify, and deliver exosomes. Various methods, such as ultracentrifugation, size exclusion chromatography, and microfluidic-based approaches, have been developed to achieve these. Additionally, various delivery methods, such as intravenous injection, inhalation, and topical application, are being explored (20).

Researchers have directed their efforts towards comprehending the functions of EVs, particularly exosomes, in the pathogenesis of COVID-19 infection. Additionally, they have explored the potential of targeting these compartments within cells as a novel therapeutic approach to COVID-19 (21). As exosome technologies hold significant promise for COVID-19 therapy, it is essential to evaluate their feasibility critically. The aim of this article is to examine the reality versus fantasy of exosome technologies and their potential role in combating COVID-19. Through an analysis of the present state of the field and considering the validity of achievements as well as addressing unrealistic expectations, we aim to provide a comprehensive assessment of the feasibility of exosome technologies in COVID-19 therapy. This evaluation will enhance the understanding of the potential of exosomes and guide future research and development in this crucial domain. Furthermore, the article elucidates various pre-clinical and contemporary clinical approaches to exosome therapy for the treatment of COVID-19, providing valuable insights into the practical aspects of their implementation.
COVID-19 pandemic overview
In December 2019, a novel strain of the coronavirus, known as COVID-19, was identified as the causative agent responsible for an unprecedented increase in cases of pneumonia in the city of Wuhan, situated in China (22,23). Rapid global dissemination has occurred (5,23). In March 2020, the World Health Organization (WHO) officially declared the COVID-19 disease as a pandemic (24). As of April 19, 2023, WHO had reported a cumulative total of 763,740,140 confirmed cases of COVID-19, along with 6,908,554 recorded deaths (25). The primary mechanisms that facilitate the transmission of COVID-19 within human populations are interpersonal contact and the airborne spread of respiratory droplets. The typical duration associated with the incubation period of COVID-19 is 2 weeks or 14 days (26). The preferred test for diagnosing COVID-19 is through the utilization of real-time reverse transcriptase-polymerase chain reaction (RT-PCR) (27,28). Patients diagnosed with COVID-19 have reported a variety of symptoms, including a high body temperature, a dry cough, breathlessness, ageusia or anosmia, diarrhoea, and nausea (5,23,29).
However, preexisting diseases like diabetes mellitus, hypertension, and cancer may increase COVID-19 mortality risk (30). The immune system acts as the primary protective response in patients affected by SARS-CoV-2, with supportive care being provided in the event of any ensuing complications (31). The availability of multiple effective vaccines, scientists worldwide are still exploring all possible treatment strategies for treating infected populations owing to the regular revelation of new information regarding the SARS-CoV-2 virus structure, virulence, and immunological characteristics (32).
Pathophysiology of COVID-19
The coronavirus belongs to the coronaviridae family and the coronavirus genus, characterized by its enveloped structure and possessing a non-segmented positive single-stranded RNA genome of varying in length typically ranges from 26–32 kb (33). The coronaviruses are composed of four fundamental structural proteins, namely the spike, membrane, and envelope, alongside nucleocapsid (Figure 2) (34). The virus utilizes two cellular receptors, ACE2 and the transmembrane serine protease 2 (TMPRS2), to enter and infect human cells (35,36). Given its broad distribution on the surface of human cells, including pulmonary, renal, cardiac, vascular, and intestinal cells, the ACE2 receptor is a major contributor to systemic diseases (37). The virus severely impacts the lungs, which are the primary infected organ, owing to the substantial upregulation of both receptors in the alveolar type-2 cells (38). The reduction of ACE2 on the surface of alveolar cells after viral invasion results in a shift in the ACE/ACE2 equilibrium towards ACE, which promotes oxidative stress and vascular inflammation (39). Upon the initial encounter, the immune system is stimulated, leading to the activation of cytotoxic cells, antibody production, and interferon release. In the later stages of COVID-19, T lymphocytes, neutrophils, and macrophages infiltrate the alveoli, where they generate cytokines such as interleukin-1, interleukin-6 (IL-1, IL-6) and tumour necrosis factor-alpha (TNF-α) (40). The occurrence of both ARDS and multi-organ dysfunction is a direct result of the cytokine storm that develops (41). The condition of hyper-inflammation is linked to a hypercoagulable state as a result of the excessive expression of tissue factors in the coagulation cascade (42).
During the process of viral attachment and entry, the S proteins of the virus engage in an interaction with the cell membrane-bound ACE2. The S protein has two distinct regions, S1 and S2, based on its molecular structure. SARS-CoV-2 infection requires subunit S1 activation to maintain virus-epithelial cell contact while the subunit S2 facilitates SARS-CoV-2 plasma membrane fusion (43). Molecular analysis indicates that SARS-CoV-2 has undergone various modifications in its genomic structure, potentially influencing its transmissibility and virulence. Specifically, the omicron variant (B.1.1.529) displays specific mutations in its S protein, including three deletions and one insertion. These alterations in the S protein have been observed to diminish the effectiveness of both locally and systemically produced neutralizing antibodies against the Omicron variant, following vaccination or prior infection, in comparison to the original SARS-CoV-2 strain (44,45). Viral pneumonia, ARDS, septic shock, and cardiovascular illness are the main causes of SARS-CoV-2 deaths. ARDS is the leading cause of death among them (46).
Current COVID-19 therapeutic interventions
Various therapeutic modalities have been utilized in individuals affected by COVID-19 to reduce the mortality rate (47,48). The primary medical approach to combat COVID-19 involves the utilisation of off-label antibiotics and antiviral medications to impede the replication cycle of the virus (49). Although some patients may be asymptomatic or exhibit mild symptoms, old age and those with pre-existing conditions like chronic pulmonary disease, diabetes mellitus, and cardiovascular disorders constitute a significant portion, ranging from 10% to 34%, of the virus-infected population and are risk of developing ARDS and multiple organ failure (50). A group of researchers has initiated a large-scale competition aimed at identifying effective solutions for the management of the highly fatal coronavirus. The endeavours of the researchers have been broadened from the utilisation of pre-existing drugs that have received approval for alternative diseases to the innovation of novel drugs and therapeutic approaches. Several drugs that have been traditionally used for other diseases have shown efficacy in treating COVID-19 in animals. Remdesivir, chloroquine (used to treat malaria), lopinavir (used to treat human immunodeficiency virus), and a combination of lopinavir, interferon-beta, and ritonavir are utilized (51,52). There are additional preventive and management measures available for COVID-19, including vaccination and convalescent plasma therapy. Nevertheless, the effectiveness of these approaches is dependent upon the stability of viral epitopes, which is compromised by the rapid mutation of RNA in this novel virus. This mutation also inhibits the function of host T cells, resulting in frequent occurrences of multi-organ failure in individuals with immunodeficiency, even those who are otherwise healthy (53). Moreover, a multitude of pharmaceutical agents, including nitazoxanide, ivermectin, corticosteroids, tocilizumab, sarilumab, and various other compounds that remain obscure, have been scrutinised by researchers and scientists globally, to discover an effective treatment for COVID-19 pandemic.
COVID-19 can be prevented by immunizations and utilization of serum transfusion from affected people (54). The efficacy of utilising commercially available neutralizing antibodies, whether applied locally or systemically, as a preventive measure against breakthrough infections appears to be limited. The administration of antibodies has limited efficacy when applied directly and is most effective within a few days following the emergence of clinical manifestation (55). It is noteworthy that, according to recent investigations, the majority of monoclonal antibodies utilized in clinical settings exhibit inactivity against omicron (56). The utilization of stem cell-based therapies has emerged as a potential treatment option in the management of COVID-19. Despite notable advancements, stem cell-based treatments continue to be constrained by various limitations such as immunogenicity, restricted cell source, and ethical considerations, which restrict their practical application in clinical settings (57).
Brief description of exosomes and their functions
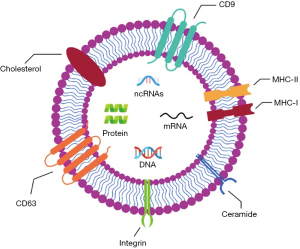
Exosomes, a distinct subset of EVs, are generated and secreted by several cellular types in both physiological and pathological conditions (58). Nano-sized particles, ranging from 40 to 150 nm, are present in diverse biofluids and play a role in intercellular communication (Figure 3). Exosomes, which are minute vesicles, are secreted by diverse cell types in reaction to varying stimuli. EVs possess the ability to transfer signalling molecules inter-cellularly and have demonstrated potential in promoting tissue regeneration, suppressing inflammation, and modulating immune cell activity. In the context of transportation, exosomes facilitate the transfer of specific signalling biomolecules that are potentially implicated in maintaining cellular hemostasis (59). In addition to the disposal of waste products, it has been demonstrated through the collection of data that exosomes exhibit distinct protein and genomic markers that are indicative of the metabolic state of the cells from which they originate (60). The formation of exosomes occurs through the process of membrane invagination within the early endosomes. Based on the findings of the molecular analysis, it is determined that a synthesis of exosomes in the cytosol requires a complex biogenesis mechanism (59,61). Exosome production in endosomes occurs via endosomal sorting complex required for transport (ECRT), ESCRT-dependent or ESCRT-independent pathways. This represents a highly regulated signaling pathway in which a variety of specialized effector molecules perform significant cellular functions. During later stages, the convergence of additional effectors, including the Soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) and GTPase systems, results in the fusion of later endosomes with the plasma membrane, thereby enabling the extracellular release of intraluminal vesicle (ILVs) (62). Like various other biological fluids, nasal discharge and airway mucus contain notable concentrations of exosomes. This implies that exosomes could potentially have a significant impact on the pulmonary system, under normal and abnormal physiological conditions (63).
Exosomes have been recognized as a potentially effective therapeutic intervention in the context of COVID-19 (64). Their regulatory effects on reactive oxygen production and antiviral responses in infected cells can mitigate cell death and apoptosis (65). Furthermore, the administration of exosomes has been observed to promote the process of endothelialization and restore the function of vascular tissue in individuals with COVID-19. This effect may be attributed to the inhibition of pro-inflammatory response and the promotion of angiogenesis. Notably, exosomes exhibit a favourable safety profile, as they have not been associated with significant adverse effects (66).
Employing exosomes as a therapeutic strategy for COVID-19
Therapeutic interventions that are efficacious for individuals affected by COVID-19 remain elusive. Despite their potential involvement in facilitating COVID-19, however, they may paradoxically provide beneficial outcomes in the management of COVID-19 (64). One approach to restricting the dissemination of viruses is to impede the uptake of exosomes by adjacent cells (67). Through the utilization of various optimized techniques, exosomes exhibit the potential to serve as a therapeutic platform for the management of diverse diseases, thereby overcoming associated challenges. Moreover, the utilization of exosomes as cell-free alternatives for the treatment of diseases and the regeneration of tissues holds great potential as a clinical application. This phenomenon can be attributed to their capacity to transport therapeutic cargo constituents while avoiding immune rejection and cellular toxicity (68). Exosomes derived from stem cells exhibit notable advantages, as they effectively utilise the anti-inflammatory and regenerative properties of their progenitor cells (69). Exosomal therapy can be customised for respiratory viral illnesses, including SARS-CoV-2, by incorporating knowledge of the origin of exosomes from native organ types. The therapeutic advantages of lung-derived exosomes have been observed to be superior to those of exogenous counterparts, such as MSC exosomes, specifically in the lungs. This presents a more precise and improved therapeutic approach (70). The utilization of exosomes as a therapeutic intervention for respiratory viral infections presents potential benefits in mitigating the cytokine storm and multi-organ dysfunction which is commonly observed in COVID-19 patients (71). An alternative strategy that could be considered involves the utilization of exosomes as “nano decoys” to sequester viruses and impede their entry into host cells (39). The utilization of exosomes as “nano decoys” that mimic cells presents a promising approach to attenuate viral infection by selectively binding and marking the virus, thereby facilitating its subsequent elimination by immune cells. The nanostructures that mimic cell membranes have effectively captured pathogens and do not exhibit a propensity toward developing membrane fusions with host cells. This characteristic enables the immune system to eliminate the captured pathogens before infection (72,73). Furthermore, it has been demonstrated that exosomes possess the ability to capture membrane-acting virulence factors, including pore-forming toxins, thereby hindering the uptake of viruses by host cells (74). The implementation of entrapment strategies can facilitate the neutralization of the host’s inflammation after viral infection. This underscores the mechanistic parallels between exosomes and viruses, and the possibility of enhancing exosomes as “nano decoys” (75). Utilizing exosomes as “nanodecoys”, these structures act as natural binders to viruses, hindering their entry into host cells (76,77). Composed of membrane proteins and receptors derived from the parent cell, exosomes possess the ability to entrap and halt viruses that would otherwise infect the host cell (77). This strategic use of exosomes, mimicking cells at a nanoscale level, has the potential to impede viral infections by capturing and marking the virus for subsequent elimination by immune cells (78). Hence, exosomes derived from the lungs exhibiting ACE2 on their surfaces, may serve as more effective decoys, given that these exosomes are of natural origin and possess the requisite cell surface ligands and receptors that enable them to efficiently attach SARS-CoV-2 and impede cellular uptake. In addition to endogenous exosomes, the utilisation of modified exosomes as vehicles for targeted drug delivery represents a promising therapeutic approach.
Exosomes surpass synthetic vesicles due to their natural capacity for targeted cargo delivery to recipient cells, using their membrane proteins and lipids. They also traverse the blood-brain barrier via endocytic pathways, facilitating substance transport to the brain (79,80). Exosomes have the potential to encapsulate miRNAs and diverse drugs. The utilisation of miRNAs or drugs has been observed to effectively target specific molecules within infected cells, thereby reducing inflammation or preventing apoptosis in pulmonary cells (81). In a study conducted by Song et al. in 2017, it was discovered that exosomes derived from MSCs contain miR-146a. The investigators noticed the administration of IL-1b to MSCs before treatment led to an augmentation of their immunomodulatory properties, which promoted the selective targeting of distinct cells (82). This was attributed to the transfer of miR-146a from the exosomes to the target cells. Exosomes possess a distinctive configuration that renders them suitable for transportation purposes, thereby enabling them to accommodate numerous therapeutic agents that can be employed in diverse pathological conditions (83).
The combination of antiviral drugs with the immunomodulatory contents of exosomes derived from MSCs represents a novel and innovative approach to the management of COVID-19 (84). Exosomes can serve as carriers for antiviral drugs, facilitating targeted delivery to specific organs, such as the lungs. Regarding COVID-19, it is possible to administer antiviral-loaded exosomes through intranasal delivery to the nasal mucosa and respiratory system. The effectiveness of previous synthetic drug delivery systems has been restricted due to their cytotoxicity, immunogenicity, and/or inefficiency in delivery (85). The encapsulation of drugs, such as miRNA within exosomes has the potential to overcome these constraints and offer enhanced targeting of recipient cells, as has been suggested in previous studies (86). In a study conducted by Gunasekaran et al. in 2020, it was proposed that cells infected with respiratory viruses produce exosomes that encapsulate viral particles (87). Hence, suppressing the production and release of exosomes from infected cells could potentially decelerate the spread of the infection. Nevertheless, exosomes exhibit promise as a therapeutic approach for the management of COVID-19, functioning as carriers for drug delivery and therapeutic agents to mitigate inflammatory responses and facilitate the regeneration of damaged tissues.
Owing to their exceptional biocompatibility, high bioavailability, and low immunogenicity, exosomes exhibit intrinsic traits that make them a potential option for drug delivery within the domain of intercellular communication. SARS-CoV-2 antiviral exosomes may suppress viral replication and associated diseases like cytokine storm syndrome and ARDS in COVID-19 patients, in addition to their intrinsic anti-inflammatory properties. Exosomes may be tailored for influenza viruses and enteroviruses through the conjugation of antiviral medications with endogenous exosomal therapeutic constituents, to address recurring lung inflammation. The utilisation of exosomes derived from lung or lung secretion has the potential to offer a specialised acellular therapeutic approach that possesses inherent antiviral properties. This approach has the potential to facilitate the regulation of virus replication and host inflammation.
Exosomes as immunomodulators in COVID-19
Exosomes, similar to viruses, share biogenesis features and transport biomolecules (88). Viruses utilize extracellular double-membrane vesicles (EDMVs) as a means to infiltrate and replicate inside host cells, as observed in SARS-CoV infections (89,90). The RNA synthesis of SARS-CoV-2 takes place within cytoplasmic vesicles resembling the rough endoplasmic reticulum, thereby aiding in the transportation and subsequent release of viral particles (91). Exosomes transmit viral and self-antigens, triggering immune reactions, they may affect circRNA/lncRNA expression, influencing host cell processes and potentially contributing to COVID-19 recurrence (92). Exosomes from respiratory syncytial virus-infected cells stimulate innate immunity by prompting airway epithelial cells and monocytes to release cytokines and chemokines (93). Coronaviruses like SARS and MERS increase pro-inflammatory cytokines and chemokines, which cause inflammation of the lungs (94). In 2020, a study conducted by Jamilloux et al. found that fast innate immune response activation in COVID-19 patients increases acute-phase reactants. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum amyloid A, and ferritin are these reactants (95). The presence of proteins linked to platelet degranulation has been detected in SARS-CoV-2 (96). Furthermore, there is a correlation between severe COVID-19 and mortality with a low platelet count (97). Additionally, SARS-CoV-2 demonstrates elevated IL-6 levels, which have the potential to affect the release of cellular proteins via EVs (98). Infection with SARS-CoV-2 causes a cellular response that causes a cytokine storm and the detection of TNF, IL-1b, and IL-6 (99). Therefore, it is plausible that exosomes in circulation may play a role in the modulation of cellular reactions, including disease severity, tissue injury, and multi-organ dysfunction, in patients with SARS-CoV-2 infection.
Exosome-mediated mechanisms of action in COVID-19 therapy
The presence of SARS-CoV-2 leads to a cytokine storm and heightened immune response. COVID-19 patient-derived exosomes exhibit increased tumour necrosis factor (TNF) and fibroblast growth factor (FGF). Additionally, plasma exosomes from COVID-19 patients also display elevated Tenascin-C (TNC) and fibrinogen-b (FGB), which activate nuclear factor kappa light chain enhancer of activated B cells (NF-kB), inducing pro-inflammatory cytokine production (100). Exosomal noncoding RNAs influence viral infection progression. TNC and FGB within exosomes from SARS-CoV-2 patients can trigger cytokine storms and micro-thrombosis. TNC and FGB could predict COVID-19 outcomes. A recent discovery highlights plasma exosomes, abundant in TNC and FGB, potentially contribute to disease advancement. (100). Certain EVs proteins associated with the antiviral response have emerged as potential biomarkers for determining the severity of COVID-19 (101).
Evaluation of scientific evidence supporting the effectiveness of exosome-based treatments for COVID-19: a focus on clinical trials
Clinical trials are crucial to understanding and guiding COVID-19 exosome-based treatment. They are essential for evaluating exosome treatments in COVID-19 patients. Moreover, they have helped researchers understand exosome-based treatment efficacy, increasing patient outcomes and disease burden. To assess exosomes’ therapeutic potential, these trials examined exosomes’ delivery mode, clinical phase, and outcome metrics. A comprehensive electronic search was conducted in various clinical trial databases, including ClinicalTrials.gov, the International Standard Randomised Controlled Trial Number (ISRCTN) registry, the International Clinical Trials Registry Platform (ICTRP), the EU Clinical Trials Register, and Cochrane Central Register of Controlled Trials. The search was focused on identifying trials related to the keywords ‘exosomes’ and ‘COVID-19’. The parameters included trial recruitment status, participant count, clinical phase status, delivery mode, results, and trial completion status. These criteria were instrumental in ensuring a comprehensive identification of trials associated with exosomes concerning COVID-19. Through this search, a selection of clinical trials listed in Table 1 was identified before June 2023.
Table 1
| Study no. | Study ID | Recruitment status | Study participants | Clinical phase | Delivery mode | Results |
|---|---|---|---|---|---|---|
| 1 | NCT05808400 | Recruiting | N=80 | Phase I | Inhalation | Not available |
| 2 | NCT04969172 | Not recruiting | N=155 | Phase II | Inhalation | Not available |
| 3 | NCT05216562 | Recruiting | N=60 | Phase II and III | Intravenous | Not available |
| 4 | NCT04902183 | Unknown | N=90 | Phase II | Inhalation | Not available |
| 5 | NCT05787288 | Recruiting | N=240 | Phase I | Inhalation | Not available |
| 6 | NCT04602442 | Unknown | N=90 | Phase II | Inhalation | Not available |
| 7 | NCT05191381 | Recruiting | N=40 | Not defined | Application of exosomes in a whole blood assay | Not available |
| 8 | NCT04798716 | Not yet recruiting | N=55 | Phase I and II | Intravenous | Not available |
| 9 | NCT04747574 | Completed | N=35 | Phase I | Inhalation | Available |
| 10 | NCT04389385 | Unknown | N=60 | Phase I | Inhalation | Not available |
| 11 | NCT05387278 | Recruiting | N=20 | Phase I | Intravenous | Not available |
| 12 | NCT04491240 | Completed | N=30 | Phase I and II | Inhalation | Available |
| 13 | NCT04623671 | Completed | N=63 | Phase II | Intravenous | Not available |
| 14 | NCT04493242 | Completed | N=102 | Phase II | Intravenous | Available |
| 15 | ISRCTN33578935 | Completed | N=64 | Phase II | Intravenous | Not available |
| 16 | EUCTR2021-002184-22-GR | Completed | N=90 | Phase I | Inhalation | Not available |
| 17 | IRCT20190101042197N2 | Recruiting | N=72 | Phase I and II | Intravenous | Not available |
| 18 | IRCT20130812014333N164 | Completed | N=8 | Phase II and III | Intravenous | Not available |
| 19 | NCT04276987 | Completed | N=7 | Phase I | Inhalation | Available |
| 20 | NCT04384445 | Not recruiting | N=20 | Phase I and II | Intravenous | Not available |
The efficacy of exosomes as a therapeutic intervention for a wide range of diseases, encompassing regenerative medicine and cancers, has been firmly established (68,102). Exosome clinical studies have grown sevenfold in the previous five years (103). Exosome-based therapies have targeted cancer, neuro degeneration, inflammation, and immunology in these trials (104). There are currently over 100 on-going clinical studies registered, exploring the therapeutic applications and potential of exosomes. Building on the previous successful utilization of exosomes in various conditions, clinical trials specifically focusing on the use of exosomes for managing COVID-19 have rapidly emerged and are now listed in the clinical trials registry. Twenty clinical trials, both ongoing and completed, involving eight research groups, have successfully conducted comprehensive clinical evaluations since the pandemic began. Notably, four of these research groups have yielded significant outcomes concentrated on the utilization of exosomes as a promising therapeutic approach for COVID-19 as shown in Figure 4.
The ongoing clinical trials provide clear evidence of the diverse approaches being pursued in exosome research. These trials involve the examination of exosomes in various forms, including injectable and inhalable formulations, among others. These experiments utilized MSCs from bone marrow or umbilical cord. These trials aim to determine the optimal dosage of exosomes for effective treatment. Moreover, these clinical trials involve the exploration of different routes of EV administration, such as intravenous or nasal, in order to identify the most effective and practical delivery method for exosome-based therapies.
Regarding the completed studies without available results, three investigations have focused on the intravenous administration of exosome-based formulations, while one study has specifically focused on the inhalation route. In one study, the participants received these formulations. The dosage entails the administration of 150 million cardio-sphere-derived cells (CDCs) within a total volume of 100 mL. This infusion is prepared using a 5% solution of Human Serum Albumin (HSA). Subjects will undergo follow-up assessments on specific days, including Days 2, 3, 7, 15, 30, 60 and 90. These assessments will be conducted either during the patient’s hospital stay or through telephone communication if they have already been discharged. The maximum duration of subject participation in the study will be 13 weeks from the initial screening (registered under NCT04623671). Similarly, in the second study administered MSC-derived exosome formulations with a dose of 0.2 mg/kg, with each dose delivered in a total volume of 15 mL, on both Day 1 and Day 3, during the Phase II clinical stage (registered under ISRCTN33578935). Moreover, in the third study, the administration of exosomes is carried out as a single dose, with a dosage of one billion exosomes per kilogram. This single dose is repeated twice on two consecutive days intravenously. The separation and processing procedures take place within the cell culture laboratory situated at the Biological Research Centre of Kermanshah University of Medical Sciences (registered under IRCT20130812014333N164). Furthermore, The Athens Medical Society has conducted a randomized, single-blind dosage study to assess the safety and effectiveness of exosomes overexpressing CD24. This clinical trial compared the outcomes between a dose of 109 and a dose of 1010 administered through inhalation (registered under EUCTR2021-002184-22-GR).
There have been three completed studies that examined the use of exosome-based formulations administered through inhalation, while one study specifically explored the administration of such formulations through the intravenous route. In a clinical trial conducted in In the Athens Medical Society (Greece) (registered under NCT04747574), exosomes engineered to overexpress CD24 were registered for use in preventing clinical deterioration in patients with moderate or severe COVID-19. These exosomes were administered once daily for 5 days via inhalation derived from human embryonic kidney T-RExTM-293 cells. The study results showed significant improvements in respiratory rate and pulse oximetry in 83.7% and 64% of the analyzed patients, respectively. Additionally, inflammatory indices levels dropped by at least 50% from baseline admission values in 82.8% of the analyzed patients on day 7. These findings indicate the potential efficacy and safety of the EXO-CD24 treatment for improving clinical and laboratory parameters in patients with moderate or severe COVID-19, warranting further investigation through a phase 3 study. Similarly, another clinical trial (NCT04491240) was conducted where participants received nanoparticles of exosomes twice a day for 10 days via inhalation. The inhalation involved a 3 mL special solution containing (0.5–2)×1010 nanoparticles (exosomes) of two different types. A placebo group received a special solution without any nanoparticles. The results of the trial demonstrated a positive impact on patients with COVID-19 pneumonia, indicating the need for further investigation through a phase 3 clinical trial to validate these findings. In another trial (registered under NCT04276987), inhalation of clinical-grade human adipose-derived mesenchymal stromal cell (haMSC) exosomes was explored in seven COVID-19 patients. Consecutive inhalation doses over 5 days, up to a total of 2.0×109 nanovesicles, were well-tolerated, with no adverse events or dose-related toxicity. Computed tomography (CT) imaging displayed improvement within seven days. However, more extensive trials are essential to validate long-term safety and effectiveness. Furthermore, another clinical trial registered under NCT04493242 evaluated the intravenous administration of EVs derived from bone marrow mesenchymal stem cells (bmMSC-EVs). The participants were divided into three groups, with two groups receiving different doses of bmMSC-EVs and the third group receiving a placebo for control. The trial demonstrated good tolerability with no reported adverse events or dose-related toxicity. However, further large-scale trials are necessary to validate the long-term safety and effectiveness of this treatment approach.
The COVID-19 situation has once again brought a lot of attention to exosomes as a potentially effective cell therapy. Researchers hope that the results of ongoing trials involving exosomes can bring hope to the field of regenerative medicine, specifically for viral pneumonia and other related conditions. Although there are only a few clinical studies available, they provide valuable information about the safety and potential effectiveness of inhaled and intravenous exosomes for managing COVID-19. However, more research is needed to determine the optimal dosage, suitable conditions for culturing exosomes, and appropriate methods of administration, and standardized protocols for isolating and storing exosomes. This will enable exosomes to be widely used in diagnosing and treating various diseases.
Identification of exaggerated claims and misconceptions in exosome technologies for COVID-19 therapy
The urgent need for effective treatments for COVID-19 has generated a significant level of interest in exosome technologies as a prospective therapeutic modality. Nevertheless, it is imperative to undertake a thorough investigation of the existing discourse about exosome-based therapies for COVID-19, given the alarming prevalence of exaggerated claims and misunderstandings within this domain. Despite the promising role of exosomes in facilitating intercellular communication and their proven efficacy in the treatment of various conditions, it is imperative to acknowledge and address the notable limitations associated with their application (70,105).
The most significant challenge involves the identification of a suitable cell source that is in line with the therapeutic goals. Several variables, such as the specific tissue from which MSCs are derived, the age of the donor, and the conditions under which they are cultured, have the potential to impact the therapeutic characteristics of these cells. These characteristics include their ability to modulate the immune response and their capacity for differentiation (106,107).
The second challenge relates to the dependency of exosome generation on cell culture, given that exosomes are released by cells. In order to achieve a substantial yield of exosomes, it is crucial to cultivate a substantial population of cells while preserving their genotypic and phenotypic traits (108). Nevertheless, the challenge of acquiring a significant quantity of cells in a cost-effective and efficient manner continues to impede the production of stable products. This limitation specifically impedes the generation of substantial quantities of stem cell-conditioned medium that contains exosomes derived from cells (109). Notwithstanding this challenge, there have been significant developments in cell culture technologies, specifically in the area of enhancing surface area, which has effectively tackled this constraint. However, the management of environmental factors and the reduction of the potential for phenotypic changes and the formation of ABs formation remain complex tasks, as they have a direct influence on both the quality and quantity of the resulting exosome product (110). The emergence of perfusion bioreactors, specifically hollow-fibre perfusion bioreactors, has shown promise in the context of exosome secretion by cells. The bioreactors mentioned in the study enable efficient mass transfer within the culture, enable the maintenance of continuous high-cell-density cultures, and effectively decrease the reliance of cells on serum factors (108). This method effectively increases the concentration of exosomes, leading to more efficient and cost-effective downstream processing (111).
Another significant constraint challenge linked to the large-scale cultivation of cells for exosome production is the dependence on animal serum to facilitate optimal cell proliferation (108,111). The utilisation of animal serum presents a heightened susceptibility to viral contamination, thereby giving rise to a significant safety concern. Consequently, this contamination would render the product unsuitable for therapeutic purposes when administered via injection. In order to tackle this matter, an alternative strategy entails the utilisation of culture media components that are devoid of serum and xenobiotic substances. Nevertheless, the task at hand is to guarantee that these substitutions uphold the therapeutic characteristics of the exosome product, reaching similar levels as those achieved with animal serum (112). Exosomes are easily isolated from the conditioned medium, unlike adhering producer cells like stem cells (109). In contrast, adherent cell products require enzymatic detachment, resulting in elevated downstream processing expenses and extended purification durations. As a result, this particular procedure leads to a decrease in overall efficiency and the possibility of a decline in the final exosome product (20).
In the field of downstream processing, the purification of exosomes typically involves the utilisation of four primary separation techniques: sedimentation force, affinity isolation, filtration, and polymeric precipitation (113). Nevertheless, it is important to note that these methods may have inherent limitations that interfere with their use in the context of producing exosomes on a larger scale. One example of a technique that has certain limitations is ultracentrifugation. This method is characterised by time-consuming procedures, involving multiple steps, and the possibility of exosome disruption during the separation process. Consequently, its feasibility for large-scale production is reduced (15). In contrast, the process of nano-specific precipitation requires supplementary downstream procedures for the removal of polyethene glycol (PEG) from the ultimate product. Moreover, this method may lead to diminished purity as a consequence of the simultaneous isolation of different categories of EVs and proteins (113,114). The absence of standardised protocols and comprehensive characterization systems presents additional challenges in the utilisation of exosomes in vivo settings (58). The maintenance of sterility and the mitigation of potentially infectious agents, such as viral particles and mycoplasma, are crucial factors that must be taken into account when conducting the exosome purification procedure (115,116).
Previously, the term “exosomes” lacked clear differentiation among various isolated EVs (117,118). The Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines, as presented by the International Society of Extracellular Vesicles (ISEV) in 2018, notably addressed the ambiguity surrounding the definition of exosome subpopulation (119). These guidelines acknowledged the limitations of existing extraction methods in distinguishing distinct exosome subtypes and emphasized the need for precise characterizations for each subtype. Despite challenges, the guidelines aimed to rectify the confusion caused by overlapping properties, such as size and markers, among different exosome subtypes (119,120). Following the 2018 guidelines, scientific literature commonly utilizes the term “exosomes” to describe small extracellular vesicles (sEVs) derived from cell membranes, characterizing them with limited replication capacity. The broader classification of “EVs” allows for specification based on measurable traits like cell origin, markers, size, density, and function (119). These EVs encompass exosomes (<100 nm), microvesicles (150–1,000 nm), and ABs (>1 µm), each exhibiting variations in size, content, generation, release mechanisms, and functions (121). Initially, exosomes regarded as carriers of cellular waste, however, they have a significant roles in facilitating intercellular communication in both normal and pathological conditions (122). These guidelines, guided by MISEV, have directed efforts to align discussions on exosomes and enhance clarity in characterizing these EVs. In addition, the quantitative assessment of the bioactive components present in the exosome product poses a considerable obstacle in the endeavour to achieve therapeutic-scale production (120).
Another significant challenge in the clinical utilisation of exosomes is their inherent pro-coagulation activity (123). The presence of certain receptors and proteins, such as phosphatidylserine and tissue factor, on the surface of exosomes, is considered to be responsible for this characteristic. These components are known to play crucial roles in facilitating coagulation activity (124,125). Several factors, such as the cellular origin of exosomes, their passage number, and viability, have been found to potentially enhance the coagulation cascade when exosomes are administered (125,126). To attenuate the procoagulant activity exhibited by exosomes, a suggested approach entails subjecting them to a treatment regimen involving a combination of anticoagulant agents, namely heparin and bivalirudin. Nevertheless, a study conducted by Silachev et al. (126) have demonstrated through their research that the use of heparin as a monotherapy is more efficacious in mitigating exosome-induced procoagulant activity compared to employing various proteins for the same purpose. Furthermore, the significance of the isolated exosome product’s quality cannot be overstated, as it plays a crucial role in reducing coagulation activity. A study conducted by Nielsen et al. demonstrated that the reduction of contaminants, such as plasma proteins and lipoproteins, through enhanced purification techniques, can effectively decrease the likelihood of thrombosis in individuals diagnosed with cancer (127). Anticoagulation and fibrinolytic drugs are often given to hospitalized COVID-19 patients owing to their higher risk of coagulopathy and venous thromboembolism (128,129). Thus, COVID-19 patients at high risk of thrombosis should be cautious when assessing exosome problems. However, Jamshidi et al. found that MSCs can reduce COVID-19-related thrombosis and coagulation. This effect is achieved through the regulation of hemostasis factors (130). MSCs utilize varied approaches, including preventing vascular endothelial cell apoptosis, curbing pro-inflammatory cytokines (TNF-α, IFN-c, IL-6), impeding micro thrombi formation by immune cells (T-cytotoxic lymphocytes, NK cells, B cells), and shifting macrophages towards M2 phenotype, promoting neovascularization and tissue repair (130,131). The exosomes secreted by MSCs are anticipated to possess advantageous properties, thereby reinforcing the potential therapeutic application of MSC-derived exosomes in the management of COVID-19 patients.
To address these limitations and misconceptions, an evidence-based discourse is required, which necessitates thorough empirical validation, standardized methodologies, and detailed characterizations. Such an approach is required to maintain scientific integrity, assist informed decision-making, and develop a more comprehensive knowledge of the potentials and restrictions inherent in exosome-based COVID-19 management. Moreover, the completion of the ongoing clinical trials is anticipated to allow a thorough meta-analysis, which will facilitate the development of an efficacious therapeutic strategy utilizing exosomes for the management of COVID-19 and other related conditions. Lastly, advancements in enhancing the potency of exosomes and scaling up their production will likely bring exosome-based therapy into practical use in the near future.
Conclusions
The unprecedented global pandemic has sparked accelerated research into exosome-based therapeutic approaches. This comprehensive review of exosome effects highlighted their involvement in COVID-19 virus transmission, infection, diagnostics, treatment, therapies, medication administration, and vaccinations. However, the feasibility of exosome technologies lies at the crossroads of reality and fantasy. While their potential for diagnostics, immunomodulation, therapeutics, and vaccine development is undeniable. Rigorous scientific investigation is essential to differentiate between promising applications and unrealistic expectations. Exosome therapies have potential properties, however, synthesis, purification, downstream processing, and utilization are challenging. To maximize exosome outputs and determine COVID-19 therapy efficacy, further investigation is required to refine exosome isolation techniques. Large-scale randomized controlled trials comparing exosomes from different sources are required to advance medicinal development. Their immunomodulatory, regenerative, and viral infection impacts may be affected by heterogeneity from varied origins, ageing, separation, purification, and functional variations. Therefore, fundamental research should refine exosome isolation, characterisation, purification, and application techniques to assess their safety and efficacy as COVID-19 treatments. A scalable production technique that meets current good manufacturing practice requirements and produces functioning exosomes for clinical usage requires collaboration between researchers and industry. Exosome use in immunological diseases, such as COVID-19, should be assessed in pre-clinical and clinical studies. Despite exosomes numerous advantages, cell-based therapies long-term safety and efficacy must be addressed. To completely utilize exosomes for human health and develop COVID-19 treatments, comprehensive investigations are required.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-204/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-204/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis 2020;11:216-28. [Crossref] [PubMed]
- Mangalmurti N, Hunter CA. Cytokine Storms: Understanding COVID-19. Immunity 2020;53:19-25. [Crossref] [PubMed]
- Gupta A, Kashte S, Gupta M, et al. Mesenchymal stem cells and exosome therapy for COVID-19: current status and future perspective. Hum Cell 2020;33:907-18. [Crossref] [PubMed]
- Murthy S, Gomersall CD, Fowler RA. Care for Critically Ill Patients With COVID-19. JAMA 2020;323:1499-500. [Crossref] [PubMed]
- Zeng Z, Yu H, Chen H, et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit Care 2020;24:525. [Crossref] [PubMed]
- Silva MJA, Ribeiro LR, Gouveia MIM, et al. Hyperinflammatory Response in COVID-19: A Systematic Review. Viruses 2023;15:553. [Crossref] [PubMed]
- Sleem A, Saleh F. Mesenchymal stem cells in the fight against viruses: Face to face with the invisible enemy. Curr Res Transl Med 2020;68:105-10. [Crossref] [PubMed]
- Thanh Le T, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov 2020;19:305-6. [Crossref] [PubMed]
- Kashte S, Maras JS, Kadam S. Bioinspired Engineering for Liver Tissue Regeneration and Development of Bioartificial Liver: A Review. Crit Rev Biomed Eng 2018;46:413-27. [Crossref] [PubMed]
- Nemeth K. Mesenchymal stem cell therapy for immune-modulation: the donor, the recipient, and the drugs in-between. Exp Dermatol 2014;23:625-8. [Crossref] [PubMed]
- Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem 2019;88:487-514. [Crossref] [PubMed]
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2:569-79. [Crossref] [PubMed]
- Saad MH, Badierah R, Redwan EM, et al. A Comprehensive Insight into the Role of Exosomes in Viral Infection: Dual Faces Bearing Different Functions. Pharmaceutics 2021;13:1405. [Crossref] [PubMed]
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020;367:eaau6977. [Crossref] [PubMed]
- Hade MD, Suire CN, Suo Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021;10:1959. [Crossref] [PubMed]
- Kiaie N, Ghanavati SPM, Miremadi SS, et al. Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Therapy, Preclinical and Clinical Evidence. Int J Stem Cells 2021;14:252-61. [Crossref] [PubMed]
- Akbari A, Rezaie J. Potential therapeutic application of mesenchymal stem cell-derived exosomes in SARS-CoV-2 pneumonia. Stem Cell Res Ther 2020;11:356. [Crossref] [PubMed]
- Raghav A, Khan ZA, Upadhayay VK, et al. Mesenchymal Stem Cell-Derived Exosomes Exhibit Promising Potential for Treating SARS-CoV-2-Infected Patients. Cells 2021;10:587. [Crossref] [PubMed]
- Alahdal M, Elkord E. Promising use of immune cell-derived exosomes in the treatment of SARS-CoV-2 infections. Clin Transl Med 2022;12:e1026. [Crossref] [PubMed]
- Chen J, Li P, Zhang T, et al. Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol 2021;9:811971. [Crossref] [PubMed]
- Hassanpour M, Rezaie J, Nouri M, et al. The role of extracellular vesicles in COVID-19 virus infection. Infect Genet Evol 2020;85:104422. [Crossref] [PubMed]
- Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol 2020;92:401-2. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed 2020;91:157-60. [PubMed]
- World Health Organization. WHO COVID-19 dashboard > Cases. 2023. Available online: https://data.who.int/dashboards/covid19/cases
- Rezaee H, Pourkarim F, Pourtaghi-Anvarian S, et al. Drug-drug interactions with candidate medications used for COVID-19 treatment: An overview. Pharmacol Res Perspect 2021;9:e00705. [Crossref] [PubMed]
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25:2000045. [Crossref] [PubMed]
- Rubin EJ, Baden LR, Morrissey S, et al. Medical Journals and the 2019-nCoV Outbreak. N Engl J Med 2020;382:866. [Crossref] [PubMed]
- Lechien JR, Chiesa-Estomba CM, Hans S, et al. Loss of Smell and Taste in 2013 European Patients With Mild to Moderate COVID-19. Ann Intern Med 2020;173:672-5. [Crossref] [PubMed]
- Nouri-Vaskeh M, Kalami N, Zand R, et al. Comparison of losartan and amlodipine effects on the outcomes of patient with COVID-19 and primary hypertension: A randomised clinical trial. Int J Clin Pract 2021;75:e14124. [Crossref] [PubMed]
- Sharma A, Tiwari S, Deb MK, et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents 2020;56:106054. [Crossref] [PubMed]
- Trivedi N, Verma A, Kumar D. Possible treatment and strategies for COVID-19: review and assessment. Eur Rev Med Pharmacol Sci 2020;24:12593-608. [PubMed]
- de Haan CA, Kuo L, Masters PS, et al. Coronavirus particle assembly: primary structure requirements of the membrane protein. J Virol 1998;72:6838-50. [Crossref] [PubMed]
- Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J 2019;16:69. [Crossref] [PubMed]
- Hoffmann M, Kleine-Weber H, Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol Cell 2020;78:779-784.e5. [Crossref] [PubMed]
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. [Crossref] [PubMed]
- Verma S, Saksena S, Sadri-Ardekani H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesis†. Biol Reprod 2020;103:449-51. [Crossref] [PubMed]
- Devaux CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect 2020;53:425-35. [Crossref] [PubMed]
- Zhang Q, Honko A, Zhou J, et al. Cellular Nanosponges Inhibit SARS-CoV-2 Infectivity. Nano Lett 2020;20:5570-4. [Crossref] [PubMed]
- Boechat JL, Chora I, Morais A, et al. The immune response to SARS-CoV-2 and COVID-19 immunopathology - Current perspectives. Pulmonology 2021;27:423-37. [Crossref] [PubMed]
- Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 2022;19:92. [Crossref] [PubMed]
- Subramaniam S, Kothari H, Bosmann M. Tissue factor in COVID-19-associated coagulopathy. Thromb Res 2022;220:35-47. [Crossref] [PubMed]
- Wang MY, Zhao R, Gao LJ, et al. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front Cell Infect Microbiol 2020;10:587269. [Crossref] [PubMed]
- Shuai H, Chan JF, Hu B, et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 2022;603:693-9. [Crossref] [PubMed]
- Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022;602:671-5. [Crossref] [PubMed]
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033-4. [Crossref] [PubMed]
- Rezabakhsh A, Ala A, Khodaei SH. Novel coronavirus (COVID-19): a new emerging pandemic threat. J Clin Med Res 2020;8:5. [Crossref]
- Gurunathan S, Kang MH, Kim JH. Diverse Effects of Exosomes on COVID-19: A Perspective of Progress From Transmission to Therapeutic Developments. Front Immunol 2021;12:716407. [Crossref] [PubMed]
- Majumder J, Minko T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J 2021;23:14. [Crossref] [PubMed]
- Lee PI, Hsueh PR. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect 2020;53:365-7. [Crossref] [PubMed]
- Kupferschmidt K, Cohen J. Race to find COVID-19 treatments accelerates. Science 2020;367:1412-3. [Crossref] [PubMed]
- Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020;323:1824-36. [Crossref] [PubMed]
- Soltani S, Zakeri AM, Karimi MR, et al. A Systematic Literature Review of Current Therapeutic Approaches for COVID-19 Patients. Journal of Pharmaceutical Research International 2020;32:13-25. [Crossref]
- Grein J, Ohmagari N, Shin D, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med 2020;382:2327-36. [Crossref] [PubMed]
- Gottlieb RL, Nirula A, Chen P, et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2021;325:632-44. [Crossref] [PubMed]
- Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022;602:671-5. [Crossref] [PubMed]
- Golchin A, Farahany TZ. Biological Products: Cellular Therapy and FDA Approved Products. Stem Cell Rev Rep 2019;15:166-75. [Crossref] [PubMed]
- Rezabakhsh A, Sokullu E, Rahbarghazi R. Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res Ther 2021;12:521. [Crossref] [PubMed]
- Heidarzadeh M, Gürsoy-Özdemir Y, Kaya M, et al. Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell Biosci 2021;11:142. [Crossref] [PubMed]
- Elzanowska J, Semira C, Costa-Silva B. DNA in extracellular vesicles: biological and clinical aspects. Mol Oncol 2021;15:1701-14. [Crossref] [PubMed]
- Gurung S, Perocheau D, Touramanidou L, et al. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 2021;19:47. [Crossref] [PubMed]
- Gurunathan S, Kang MH, Kim JH. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int J Nanomedicine 2021;16:1281-312. [Crossref] [PubMed]
- Cha S, Seo EH, Lee SH, et al. MicroRNA Expression in Extracellular Vesicles from Nasal Lavage Fluid in Chronic Rhinosinusitis. Biomedicines 2021;9:471. [Crossref] [PubMed]
- Gurunathan S, Kang MH, Kim JH. Diverse Effects of Exosomes on COVID-19: A Perspective of Progress From Transmission to Therapeutic Developments. Front Immunol 2021;12:716407. [Crossref] [PubMed]
- Rezabakhsh A, Mahdipour M, Nourazarian A, et al. Application of exosomes for the alleviation of COVID-19-related pathologies. Cell Biochem Funct 2022;40:430-8. [Crossref] [PubMed]
- Amirzadeh Gougheri K, Ahmadi A, Ahmadabadi MG, et al. Exosomal Cargo: Pro-angiogeneic, anti-inflammatory, and regenerative effects in ischemic and non-ischemic heart diseases - A comprehensive review. Biomed Pharmacother 2023;168:115801. [Crossref] [PubMed]
- Schneider DJ, Speth JM, Penke LR, et al. Mechanisms and modulation of microvesicle uptake in a model of alveolar cell communication. J Biol Chem 2017;292:20897-910. [Crossref] [PubMed]
- Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant 2019;54:789-92. [Crossref] [PubMed]
- Rani S, Ryan AE, Griffin MD, et al. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther 2015;23:812-23. [Crossref] [PubMed]
- Popowski KD, Dinh PC, George A, et al. Exosome therapeutics for COVID-19 and respiratory viruses. View (Beijing) 2021;2:20200186. [Crossref] [PubMed]
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [Crossref] [PubMed]
- Rao L, Tian R, Chen X. Cell-Membrane-Mimicking Nanodecoys against Infectious Diseases. ACS Nano 2020;14:2569-74. [Crossref] [PubMed]
- Rao L, Xia S, Xu W, et al. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc Natl Acad Sci U S A 2020;117:27141-7. [Crossref] [PubMed]
- Keller MD, Ching KL, Liang FX, et al. Decoy exosomes provide protection against bacterial toxins. Nature 2020;579:260-4. [Crossref] [PubMed]
- de Carvalho JV, de Castro RO, da Silva EZ, et al. Nef neutralizes the ability of exosomes from CD4+ T cells to act as decoys during HIV-1 infection. PLoS One 2014;9:e113691. [Crossref] [PubMed]
- Li Z, Wang Z, Dinh PC, et al. Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat Nanotechnol 2021;16:942-51. [Crossref] [PubMed]
- Zhang Q, Honko A, Zhou J, et al. Cellular Nanosponges Inhibit SARS-CoV-2 Infectivity. Nano Lett 2020;20:5570-4. [Crossref] [PubMed]
- Popowski KD, Dinh PC, George A, et al. Exosome therapeutics for COVID-19 and respiratory viruses. View (Beijing) 2021;2:20200186. [Crossref] [PubMed]
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. [Crossref] [PubMed]
- Sen S, Xavier J, Kumar N, et al. Exosomes as natural nanocarrier-based drug delivery system: recent insights and future perspectives. 3 Biotech 2023;13:101.
- Shah D, Das P, Alam MA, et al. MicroRNA-34a Promotes Endothelial Dysfunction and Mitochondrial-mediated Apoptosis in Murine Models of Acute Lung Injury. Am J Respir Cell Mol Biol 2019;60:465-77. [Crossref] [PubMed]
- Song Y, Dou H, Li X, et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017;35:1208-21. [Crossref] [PubMed]
- Vader P, Mol EA, Pasterkamp G, et al. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev 2016;106:148-56. [Crossref] [PubMed]
- Gupta A, Kashte S, Gupta M, et al. Mesenchymal stem cells and exosome therapy for COVID-19: current status and future perspective. Hum Cell 2020;33:907-18. [Crossref] [PubMed]
- Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020;181:1016-1035.e19. [Crossref] [PubMed]
- Elsharkasy OM, Nordin JZ, Hagey DW, et al. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev 2020;159:332-43. [Crossref] [PubMed]
- Gunasekaran M, Bansal S, Ravichandran R, et al. Respiratory viral infection in lung transplantation induces exosomes that trigger chronic rejection. J Heart Lung Transplant 2020;39:379-88. [Crossref] [PubMed]
- Crenshaw BJ, Gu L, Sims B, et al. Exosome Biogenesis and Biological Function in Response to Viral Infections. Open Virol J 2018;12:134-48. [Crossref] [PubMed]
- Martins ST, Alves LR. Extracellular Vesicles in Viral Infections: Two Sides of the Same Coin? Front Cell Infect Microbiol 2020;10:593170. [Crossref] [PubMed]
- Badierah RA, Uversky VN, Redwan EM. Dancing with Trojan horses: an interplay between the extracellular vesicles and viruses. J Biomol Struct Dyn 2021;39:3034-60. [Crossref] [PubMed]
- Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 2020;55:2000607. [Crossref] [PubMed]
- Wu Y, Zhao T, Deng R, et al. A study of differential circRNA and lncRNA expressions in COVID-19-infected peripheral blood. Sci Rep 2021;11:7991. [Crossref] [PubMed]
- Chahar HS, Corsello T, Kudlicki AS, et al. Respiratory Syncytial Virus Infection Changes Cargo Composition of Exosome Released from Airway Epithelial Cells. Sci Rep 2018;8:387. [Crossref] [PubMed]
- Coperchini F, Chiovato L, Croce L, et al. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 2020;53:25-32. [Crossref] [PubMed]
- Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev 2020;19:102567. [Crossref] [PubMed]
- Zheng Y, Zhang Y, Chi H, et al. The hemocyte counts as a potential biomarker for predicting disease progression in COVID-19: a retrospective study. Clin Chem Lab Med 2020;58:1106-15. [Crossref] [PubMed]
- Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta 2020;506:145-8. [Crossref] [PubMed]
- Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020;181:1489-1501.e15. [Crossref] [PubMed]
- Chen W. A potential treatment of COVID-19 with TGF-β blockade. Int J Biol Sci 2020;16:1954-5. [Crossref] [PubMed]
- Sur S, Khatun M, Steele R, et al. Exosomes from COVID-19 Patients Carry Tenascin-C and Fibrinogen-β in Triggering Inflammatory Signals in Cells of Distant Organ. Int J Mol Sci 2021;22:3184. [Crossref] [PubMed]
- Hanna R, Dalvi S, Sălăgean T, et al. Understanding COVID-19 Pandemic: Molecular Mechanisms and Potential Therapeutic Strategies. An Evidence-Based Review. J Inflamm Res 2021;14:13-56. [Crossref] [PubMed]
- Kalluri R. The biology and function of exosomes in cancer. J Clin Invest 2016;126:1208-15. [Crossref] [PubMed]
- Pirisinu M. The Long Journey of Extracellular Vesicles towards Global Scientific Acclamation. Adv Pharm Bull 2023;13:489-501. [Crossref] [PubMed]
- NIH. National Library of Medicine 2023 [cited 2023 5-7-2023]. Available online: https://www.clinicaltrials.gov/
- Kennedy TL, Russell AJ, Riley P. Experimental limitations of extracellular vesicle-based therapies for the treatment of myocardial infarction. Trends Cardiovasc Med 2021;31:405-15. [PubMed]
- Gao F, Chiu SM, Motan DA, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 2016;7:e2062. [Crossref] [PubMed]
- Hassanzadeh A, Rahman HS, Markov A, et al. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res Ther 2021;12:297. [Crossref] [PubMed]
- Whitford W, Guterstam P. Exosome manufacturing status. Future Med Chem 2019;11:1225-36. [Crossref] [PubMed]
- Colao IL, Corteling R, Bracewell D, et al. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol Med 2018;24:242-56. [Crossref] [PubMed]
- Gurunathan S, Kang MH, Jeyaraj M, et al. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019;8:307. [Crossref] [PubMed]
- Lai JJ, Chau ZL, Chen SY, et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv Sci (Weinh) 2022;9:e2103222. [Crossref] [PubMed]
- Kang JY, Oh MK, Joo H, et al. Xeno-Free Condition Enhances Therapeutic Functions of Human Wharton's Jelly-Derived Mesenchymal Stem Cells against Experimental Colitis by Upregulated Indoleamine 2,3-Dioxygenase Activity. J Clin Med 2020;9:2913. [Crossref] [PubMed]
- Clos-Sansalvador M, Monguió-Tortajada M, Roura S, et al. Commonly used methods for extracellular vesicles' enrichment: Implications in downstream analyses and use. Eur J Cell Biol 2022;101:151227. [Crossref] [PubMed]
- Chen YS, Lin EY, Chiou TW, et al. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi 2020;32:113-20. [PubMed]
- Rohde E, Pachler K, Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy 2019;21:581-92. [Crossref] [PubMed]
- Liu Y, Defourny KAY, Smid EJ, et al. Gram-Positive Bacterial Extracellular Vesicles and Their Impact on Health and Disease. Front Microbiol 2018;9:1502. [Crossref] [PubMed]
- Chen J, Li P, Zhang T, et al. Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol 2021;9:811971. [Crossref] [PubMed]
- Tkach M, Kowal J, Théry C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond B Biol Sci 2018;373:20160479. [Crossref] [PubMed]
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. [Crossref] [PubMed]
- Willms E, Cabañas C, Mäger I, et al. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol 2018;9:738. [Crossref] [PubMed]
- de la Torre Gomez C, Goreham RV, Bech Serra JJ, et al. "Exosomics"-A Review of Biophysics, Biology and Biochemistry of Exosomes With a Focus on Human Breast Milk. Front Genet 2018;9:92. [Crossref] [PubMed]
- Shanmuganathan M, Vughs J, Noseda M, et al. Exosomes: Basic Biology and Technological Advancements Suggesting Their Potential as Ischemic Heart Disease Therapeutics. Front Physiol 2018;9:1159. [Crossref] [PubMed]
- Chaudhary PK, Kim S, Kim S. Shedding Light on the Cell Biology of Platelet-Derived Extracellular Vesicles and Their Biomedical Applications. Life (Basel) 2023;13:1403. [Crossref] [PubMed]
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020;367:eaau6977. [Crossref] [PubMed]
- Tripisciano C, Weiss R, Eichhorn T, et al. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci Rep 2017;7:6522. [Crossref] [PubMed]
- Silachev DN, Goryunov KV, Shpilyuk MA, et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019;8:258. [Crossref] [PubMed]
- Nielsen T, Kristensen AF, Pedersen S, et al. Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation. J Extracell Vesicles 2018;7:1454777. [Crossref] [PubMed]
- Mancia G, Rea F, Ludergnani M, et al. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med 2020;382:2431-40. [Crossref] [PubMed]
- Smith T, Bushek J, LeClaire A, et al. COVID-19 Drug Therapy. Elsevier. 2020 May 14. Available online: http://repository.phb.ac.id/776/1/COVID-19-Drug-Therapy_5.14.2020.pdf
- Jamshidi E, Babajani A, Soltani P, et al. Proposed Mechanisms of Targeting COVID-19 by Delivering Mesenchymal Stem Cells and Their Exosomes to Damaged Organs. Stem Cell Rev Rep 2021;17:176-92. [Crossref] [PubMed]
- Zhang J, Kong X, Jin X, et al. Bone marrow stromal cells transplantation promotes the resolution and recanalization of deep vein thrombosis in rabbits through regulating macrophage infiltration and angiogenesis. J Cell Biochem 2019;120:11680-9. [Crossref] [PubMed]
Cite this article as: Memon MA, Shahidan WNS. Evaluating the feasibility of exosome technologies in COVID-19 treatment: navigating the intersection of reality and fantasy perspectives. AME Med J 2024;9:25.